ORIGINAL RESEARCH
Diagnostic study of primary health care products processing structure
Darlyani Mariano da SilvaI; Camila Eugenia RoseiraII; Isis Pienta Batista Dias PassosIII; Ana Paula Mhirdaui SanchesIV; Rosely Moralez de FigueiredoV
I
Nursing Student. Federal University of São Carlos. São Carlos, São Paulo,
darlyufscar@yahoo.com.br
II
Nurse. Master in Nursing. Graduate Program in Nursing. Federal University
of São Carlos. São Carlos, Sao Paulo, Brazil. E-mail: c_roseira@yahoo.com
III
Nurse. Master in Nursing. Graduate Program in Nursing. Federal University
of São Carlos. São Carlos, São Paulo, Brazil. E-mail: isispienta@gmail.com
IV
Nurse. Federal University of São Carlos. São Carlos, São Paulo, Brazil.
E-mail: anapaula_ms10@hotmail.com
V
Nurse. PhD. Associate Professor. Nursing Department. Federal University of
São Carlos. São Carlos, São Paulo, Brazil. E-mail: rosely@ufscar.br
DOI: http://dx.doi.org/10.12957/reuerj.2016.9369
ABSTRACT
Objective: to characterize the health care product processing structure and sites at all 29 primary health care facilities in a town in Sao Paulo State. Method: this quantitative descriptive study, conducted in 2012, applied primary health care structure indicators. Results: poor structure compliance (below 75%) was found, especially for lack of exclusive space or proper flow for processing, records of standards and routines and preventive maintenance of equipment; in addition, inappropriate packaging was found. All staff involved had been adequately trained for this activity. Conclusion: the physical structure for processing of health care products falls short of recommendations and the use of specific, validated indicators enabled results to be measured.
Keywords: Sterilization; indicators of health services; structure of services, quality of health care.
INTRODUCTION
In order to control and prevent health care associated infections (HAIs), government agencies have been developing policies for this purpose, since infection risks are present in any health service1. The quality in processing of health products is one of the pillars for the control and prevention of HAIs, as the absence or inadequate realization thereof can cause infections2. The main purpose of processing health products is to avoid any adverse events arising from their use3 .
To assess control and prevention of HAIs, indicators may be used which are variable measures that identify desirable or undesirable outcomes of practices and establish compliance rates. Among the dimensions assessed by them there is the structure, which evaluates the ability of human and material resources in providing quality health care2.
By considering the particularities of the Primary Health Care (PHC), applying a validated instrument for structural assessment of quality in processing of health products in the Sterilization and Material Center (SMC)4 is of utmost importance, comprising the specificities of PHC, such as equipment, health actions and space organization.
Given the above, this study aimed to characterize the structure as the processing settings of health products in PHC units of São Carlos - SP.
LITERATURE REVIEW
Unlike the hospital, there is a great diversity in the types of equipment, dimensions and health actions carried out in PHM, including in the SMC. According to the Brazilian Health Surveillance Agency (ANVISA) PHC can be classified as Class I, in which there is the processing of non-complex health products4.
The literature indicates that both hospital and ambulatory spaces have faced problems related to the structure of SMC. A study conducted in a hospital of Goiás State on Architectural standards of SMC reported that most of them did not meet the recommendations of the Collegiate Board Resolution of ANVISA (DRC) No. 50, which deals with the Technical Regulations for planning, scheduling, preparation and evaluation of physical projects of health care facilities, and they showed inadequacies such as cracks in walls5.
Parallel to this, a research conducted in large and medium-sized hospitals of the same state found inadequacies regarding the standards established by the Ministry of Health for the physical structure of SMC, among which there were the lack of technical barrier between dirty and clean areas, lack of resources for hand hygiene and lack of suitable settings for each step of processing of health products6.
A study carried out in primary health care in the city of Cuiabá-MT showed structural inadequacies for the SMC, in which 50% of basic health units analyzed did not have specific room for sterilization of health products7.
This review confirms the low scientific production on infection control in extra-hospital environment8. Moreover, it stresses the relevance of this study to better understand the issues related to the processing of health products in the PHC scenario.
METHODOLOGY
This is a descriptive study with a quantitative approach, conducted in the city of the central region of São Paulo state, with about 220,000 inhabitants9.
All health facilities of PHC were used as data collection field, which were 12 Basic Health Units (BHU) and 17 Family Health Units (FHU), however, four FHU shared the physical structure to each other, two and two, which amounted to the evaluation of 27 physical structures.
Data collection occurred from September to November 2012 through systematic field observation by using two indicators validated for structural evaluation of SMC in PHC4, lasting about 20 minutes in each unit visited. The first indicator evaluated the technical and operational resources for health products cleaning (L1), and the second indicator evaluated the technical and operational resources for the preparation, packaging, disinfection/sterilization, storage and distribution of these products (PE 5). Both had the ideal value of 100%. However, in order to better characterize the compliance of the municipality with respect to the structure, researchers used the scoring system provided by the Health Department of the UK, in which there are compliance categories according to the score: minimum compliance, up to 75%; partial compliance, between 76 and 84%; and adequate compliance, from 85%10.
This study did not involve the participation of human beings as research subjects, the assessment by the Research Ethics Committee in Human Beings was not necessary. The proposed study was authorized by the Municipal Health Department of the municipality, under Opinion No. 11/2012.
RESULTS
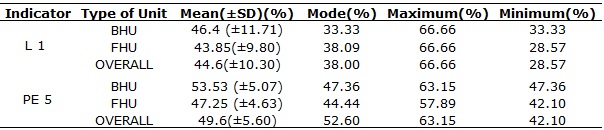
All health units, 27 (100%), in general, obtained less than the minimum value, as shown in Table 1.
TABLE 1:
Analysis of compliance rates of cleaning indicators (L1) and material
preparation/sterilization (PE 5) according to the type of unit. São Carlos
(SP), 2012.

With respect to the physical space, only 7 (25.92%) health units had exclusive area for purging (L1) and 8 (29.63%) had exclusive area for preparing, packaging and sterilization of products (PE 5). Among the units in which there was no physical separation between dirty and clean area, 7 (25.92%) had the dirty area in the same environment as the dressing room, and 8 (29.63%) had the SMC in the same environment in which dressings were performed.
In parallel, in 10 (37.03%) health units the dirty area was separated from the clean area by physical structure, whereas the concept of technical barrier was used in 8 (29.63%) health units, with a directional flow for the processing of products.
Regarding the availability of material and human resources related to the processing of health products, in relation to health products cleaning, there were soft-bristle brushes in only 6 (22.22%) health units.
In the area PE 5, there were no magnifying lenses in any unit, while kraft paper was the predominately used type of packaging (100%).
Most units, 19 (70.37%), did not have exclusive place to store the products, away from water sources, open windows or exposed pipes. After being sterilized, they were sent directly to the place of use.
For chemical disinfection, all units used solutions approved by ANVISA.
All units had steam autoclave for sterilization and used water treated by the reverse osmosis system, but these were not validated. As for the availability of personal protective equipment (PPE), only 4 (14.81%) units provided thick rubber gloves, 7 (25.92%) units provided individual goggles, 4 (14.81%) units provided facemasks and 2 (7.40%) units provided long waterproof aprons. Of the total, 23 (85.18%) had no specific place to store PPE.
As for the proper disposal of sharps and organic residues, respectively, 15 (55.55%) and 14 (51.85%) units did it correctly, however, all units lacked of available written guidance on the procedure flow after exposure to biological material by a team member.
Only 15 units (55.55%) had materials for handwashing in the setting for processing of products.
There were no records relating to the rules and routines of the areas L1 and PE 5, to the maintenance of equipment and corroborative reports of water treatment that serves the autoclaves in 100% of the units.
All (100%) professionals working in processing of products have these activities regulated by their professional councils.
DISCUSSION
On the physical space for processing health products, as found in another study that evaluated the SMC structure in hospitals of the State of Goiás, authors was observed that the suitability of the area intended for this procedure was bellow the recommended also in PHC5.
In the latter scenario, properties with household characteristics are often used for installation of health facilities, which reinforces the low valuation of structure in PHC units11.
The technical barrier concept, i.e., unidirectional flow of the dirty-and-clean process used across sectors, in a class I SMC12 was observed in only 8 (29.63%) units, which suggests that these materials, once cleaned, can be recontaminated from contact with the same surface that housed the dirty material. As to the place of storage of sterile products, studies have related storage conditions to maintenance of sterility13, 14. In one of these there was an express contamination of Enterococcus faecalis in 395 samples of steel dies, and these were divided in groups and packaged with different wrappers, among which the kraft paper. After sterilization, some were stored in closed plastic boxes and others were put loose in a closet. After successive microbiological analysis it was observed that all maintained sterility up to 148 days, in the observed conditions14.
An integrative review did not find any relationship between the relative humidity and temperature with the contamination of packaged health products, concluding that the environmental control of these variables does not affect the sterility thereof13. In the present study, 19 (70.37%) units had no exclusive location to store sterilized products.
Contrary to the Brazilian standard12, kraft paper was used for packaging in 100% of units studied; however, as found in another study14, there is no claim that this practice alone is a factor that influences the sterility of health products during storage. There are still knowledge gaps on this theme and this can be an important topic for future studies.
As regards the material and human resources related to processing of health products the use of magnifying lenses is recommended in the preparation area, with at least eight times of magnification12, which was contrary to that found in this study, since in 100% of the units professionals performed only visual inspection. A survey showed that the shortage of material and human resources was recognized by nursing professionals as generating situation of occupational stress on the SMC, in addition to interfering in the work process15.
Thus, job insecurity has affected most workers by deregulation and loss of labor rights and this may result in vulnerability to occupational diseases16.
The low availability of PPE can let the professional who performs the processing of health products vulnerable to biological risk, since it is one of the main preventive measures to do this activity. Parallel to this, among the aspects that may negatively influence the use of PPE by professionals there are discomfort, poor appearance and loss of sensitivity to handle health products17, 18.
Moreover, a study conducted in hospitals19 found that even when rubber gloves were available (90.5%) and its importance was considered by professionals (61.9%), there was negligence in using them (only 33.3% did it). Similarly, the same study revealed that knowledge about the importance of other PPE and its availability - such as glasses, cap and masks - did not ensure adherence to this type of protection.
In the present investigation, it was found that the availability of PPE for processing health products is scarce in general; however, according to the literature found, one cannot say that this single factor interfere with adherence of these by the professional.
Study found that the majority of occupational accidents were caused by sharp objects, reaching 64%20. Parallel to this, 21.6% of accidents with sharps occurred in a hospital in Minas Gerais, Brazil, were motivated by improper disposal of these objects21.
Inadequacy and/or unavailability of sites for disposal of sharps have been highlighted in several studies as one of the factors associated with the occurrence of occupational accidents22-26. It is worth remembering that sharps must be discarded in an appropriate place, before the product is sent to the SMC. This, of course, is a requirement of good practices for infection control and should be part of in-service educational activities of the whole staff.
There was no procedure flow available for professionals who suffer occupational accidents involving sharps; it was difficult to access. According to the current legislation27, all occupational accidents, regardless of severity, should be notified by recording it in the Work Accident Communication (WAC) and assessed as for indication of post-exposure measures.
Another important aspect regarding good practices for infection control is hand hygiene (HH). A research28 reported that only 56.1% of the units surveyed had specific sinks for HH, similar to data found in this study (55.55%). The same survey revealed that a large number of professionals (43.9%) had no access to their own sinks for HH and only a third of the team performed the correct HH28. The unavailability of resources required for the HH in PE setting may discourage professionals responsible for this practice to perform this technique, favoring recontamination of sterilized instruments.
According to current regulations, both the monitoring and the control of all stages of the processing of health products must be recorded, i.e., cleaning, sterilization and disinfection, and the service must keep these records in order to monitor the processing of health products. These documents must be kept for at least five years12. So the municipality needs to conform to this component of the regulation, since these records were not found in any of the units.
A work surveyed the environmental conditions of SMCs through indicators of actual temperature and relative humidity and found that the six participating hospitals were with the temperature higher than that set by NR (Regulatory Norm) No. 17 for team members' comfort (between 20 and 23° C); however, the relative humidity was in compliance with that regulation (over 40%). It is noteworthy that one of these SMCs had air conditioning system, and that even so, this did not provide the necessary thermal comfort29. In the present study authors observed absence of air-conditioned environment in all units. A study conducted with nursing professionals of a hospital SMC found that exposure to physical risks due to high temperatures and poor ventilation does not only affect the workers' health, but also the storage of materials15.
According to NR No. 15, services that perform processing of health products must have a standard operating procedure (SOP), developed according to updated scientific framework and relevant regulation, which must be disclosed to all professionals and be available for consultation12. This was not found in this study.
A study conducted in basic health units in the city of Cuiaba points to the need for investments in adaptation and enhancement of infrastructure as potentiating element of practices, and the need for investment in permanent education, which enables the expansion of practices and infrastructure in traditional BHUs8.
Contradicting a survey where employees without specific training in nursing area were observed while performing the processing of health products, a fact that interferes with the quality thereof30, in all units analyzed in this study, the professionals responsible for this activity were qualified for this task (nursing technicians or assistants and dental health assistants).
CONCLUSION
The results allowed evaluating the physical structure intended for processing of health products in PHC; it is below the desired, once the identified compliance was minimal (75%) for both the area intended for cleaning and for sterilization, storage and distribution of products.
The exclusive space for SMC is not a tangible reality, as very few health units had it. As for material resources, the most relevant data point to the need for provision of resources for the activities taking place in this space, because few SMCs had soft-bristle brushes, PPE, and about half of the units did not have adequate container for disposal of contaminated and sharp material, nor resources for HH. No unit had packages as recommended by legislation, protocols for processing health products and records of this activity or documents to ensure the validation of autoclaves. Moreover, all professionals in charge of performing the processing of health products had this activity regulated by their professional council.
A limitation of this study was the difficulty for further discussion due to reduced production in literature addressing the theme structure of SMC, especially in the PHC. However, the use of validated and specific instruments for PHC enabled the expansion of technical production on HAIs control in PHC and it is hoped that the dissemination of such results will stimulate further studies in other regions of the country, aimed at the expansion of knowledge about this theme.
REFERENCES
1.Kawagoe JY, O Centro de material e esterilização e a prevenção e o controle de infecção. In: Graziano KU, Silva A, Psaltikidis EM, organizadoras. Enfermagem em Centro de Material e Esterilização. São Paulo: Manole; 2011. p. 355-89.
2.Graziano KU, Lacerda RA, Turrini RTN, Bruna CQM, Silva CPR, Schmitt C et al . Indicadores de avaliação do processamento de artigos odonto-médico-hospitalares: elaboração e validação. Rev. esc. enferm. USP. [Internet]. 2009 [cited 2016 Feb 25]; 43(spe2):1174-1180. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0080-62342009000600005&lng=en . http://dx.doi.org/10.1590/S0080-62342009000600005
3.Padoveze MC, Graziano KU, Aspectos conceituais e microbiológicos relacionados ao processamento de materiais utilizados na assistência à saúde. In: Graziano KU, Silva A, Psaltikidis E M, organizadoras. Enfermagem em centro de material e esterilização.. São Paulo: Manole; 2011. p. 22-61.
4.Passos IPBD, Padoveze MC, Roseira CE, Figueiredo RM. Adaptação e validação de indicadores para o processamento de produtos na atenção primária à saúde. Rev. Latino-Am. Enfermagem. [Internet]. 2015 [cited 2016 Oct 1]; 23(1): 148-154. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-11692015000100148&lng=en . http://dx.doi.org/10.1590/0104-1169.3518.2536
5.Guadagnin SVT, Tipple AFV, Souza ACS. Avaliação arquitetônica dos centros de material e esterilização de hospitais do interior do estado de Goiás. Revista Eletrônica de Enfermagem [Internet]. 2007; [cited 2016 Feb 25]; 9(3):656-73. Available from: http://www.fen.ufg.br/revista/v9/n3/v9n3a07.htm
6. Guadagnin SVT, Primo MGB, Tipple AFV, Souza ACS. Centro de Material e Esterilização: Padrões Arquitetônicos e o Processamento de Artigos. Revista Eletrônica de Enfermagem. [Internet]. 2005; [cited 2016 Feb 25]; 7(3):285-93. Available from: http://www.revistas.ufg.br/fen/article/view/905 . http://dx.doi.org/10.5216/ree.v7i3.905
7.Pedrosa ICF. A infraestrutura de unidades básicas de saúde do município de Cuiabá-MT e sua relação com as práticas do enfermeiro [master dissertation]. Cuiabá (MT): Universidade Federal de Mato Grosso; 2011.
8.Figueiredo RM, Maroldi MAC. Internação domiciliar: risco de exposição biológica para a equipe de saúde. Rev. esc. enferm. USP. [Internet]. 2012 [cited 2016 Feb 25]; 46( 1 ): 145-50. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0080-62342012000100020&lng=en . http://dx.doi.org/10.1590/S0080-62342012000100020
9.Instituto brasileiro de geografia e estatística [site de internet]. Cidades. [citado em 28 fev 2016] Available from: http://www.ibge.gov.br/cidadesat/painel/painel.php?codmun=354890
10.Infection Control Nurses Association. Audit tools for monitoring infection control guidelines within the community setting [internet] 2005 [cited 2016 Jan 08] ICNA, England, p. 49. Available from: www.healthcareinformed.com/ufiles/5dbcd95a47c5/AuditTools2005
11.Moura BLA, Cunha RC, Fonseca ACF, Aquino R, Medina MG, Vilasbôas ALQ et al . Atenção primária à saúde: estrutura das unidades como componente da atenção à saúde. Rev. Bras. Saude Mater. Infant. [Internet]. 2010 Nov [cited 2013 June 25]; 10(suppl 1): s69-s81. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1519-38292010000500007&lng=en . http://dx.doi.org/10.1590/S1519-38292010000500007
12.Agência Nacional de Vigilância Sanitária (BR). Resolução no 15, de 15 de março de 2012. Dispõe sobre requisitos de boas práticas para o processamento de produtos para saúde e dá outras providências. Brasília (DF): ANVISA; 2012.
13. Bruna CQM, Graziano KU. Temperatura e umidade no armazenamento de materiais autoclavados: revisão integrativa. Rev Esc Enferm USP. [Internet]. 2012 [cited 2016 Feb 25]; 46(5): 1215-20. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0080-62342012000500025&lng=en . http://dx.doi.org/10.1590/S0080-62342012000500025
14. Serratine ACP, Gonçalves CS, Lucolli IC. Influência do armazenamento e da embalagem na manutenção da esterilidade do instrumental odontológico. Revista eletrônica de enfermagem. [Internet] 2009. [cited 2016 Feb 16]; 11(1):158-164. Available from: http://www.fen.ufg.br/revista/v11/n1/v11n1a20.htm
15. Costa CCP, Souza NVDO, Silva PAS, Oliveira EB, Vieira MLC. O trabalho na central de material: repercussões para a saúde dos trabalhadores de enfermagem. Revista Enfermagem UERJ. [Internet] 2015. [cited 2016 Feb 1]; 23(4), 533-9. Available from: www.facenf.uerj.br/v23n4/v23n4a16.pdf
16. Gonçalves FGA, Souza NVDO, Pires AS, Santos DM, D'Oliveira CAFB, Ribeiro LV. Modelo neoliberal e suas implicações para a saúde do trabalhador de enfermagem. Revista enfermagem UERJ. [Internet] 2014. [cited 2016 Feb 2]; 22:519-25 http://www.facenf.uerj.br/v22n4/v22n4a14.pdf
17.Ribeiro RP, Vianna LAC Uso dos equipamentos de proteção individual entre Trabalhadores das centrais de material e esterilização. Ciênc. cuid. saúde. Maringá. [Internet] 2012. [cited 2016 Feb 25]; 11:199-203. Available from: http://dx.doi.org/10.4025/cienccuidsaude.v11i5.17076
18.Espindola MCG, Fontana RT. Riscos ocupacionais e mecanismos de autocuidado do trabalhador de um centro de material e esterilização. Rev Gaúcha Enferm. [Internet]. 2012 [cited 2016 Feb 25]; 33(1): 116-23. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1983-14472012000100016&lng=en . http://dx.doi.org/10.1590/S1983-14472012000100016
19.Tipple AFV,Aguliari HT, Souza ACS, Pereira MS, Mendonça ACC, Silveira C. Equipamentos de proteção em centros de material e esterilização: disponibilidade, uso e fatores intervenientes à adesão. Ciênc cuid saúde Maringá. [Internet]. 2007 [cited 2016 Feb 25]; 6(4):441-8 Available from: http://www.periodicos.uem.br/ojs/index.php/CiencCuidSaude/article/view/3877
20. Bakkea HA, Araújo NMC. Acidentes de trabalho com profissionais de saúde de um hospital universitário. Prod. [Internet] 2010; [cited 2016 Feb 25]; 20:669-76. Available from: http://dx.doi.org/10.1590/S0103-65132010005000015
21.Moura JP, Gir E, Canini SRMS. Acidentes ocupacionais com material perfurocortante em um hospital regional de minas gerais, Brasil. Cienc. enferm Cienc. enferm. [Internet]. 2006 [cited 2016 Feb 25]; 12(1): 29-37. Available from: http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0717-95532006000100004&lng=es . http://dx.doi.org/10.4067/S0717-95532006000100004
22. Câmara PF, Lira C, Santos Jr BJ, Villela TAS, Heinrichsen SL. Investigação de acidentes biológicos entre profissionais da equipe multidisciplinar de um hospital. Revista Enfermagem UERJ. [Internet] 2011. [cited 2016 Feb 25];19(4)583-6 Available from: www.facenf.uerj.br/v19n4/v19n4a13.pdf
23. Silva TR, Rocha SA, Ayres JA, Juliani CMCM. Acidente com material perfurocortante entre profissionais de enfermagem de um hospital universitário. Rev Gaúcha Enferm. (Online) [Internet]. 2010 Dec [cited 2016 Feb 29]; 31(4): 615-22. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1983-14472010000400002&lng=en . http://dx.doi.org/10.1590/S1983-14472010000400002
24.Ribeiro AS, Gabatz, RIB, Neves ET, Padoin SMM. Caracterização de acidente com material perfurocortante e a percepção da equipe de enfermagem. Cogitare Enferm. [Internet]. 2009 [cited 2016 Feb 25]; 14(04):660-6. Available from: http://revistas.ufpr.br/cogitare/article/view/16379
25.Pinheiro J, Zeitoune RCG. Hepatite b: conhecimento e medidas de biossegurança e a saúde do trabalhador de enfermagem. Esc Anna Nery. [Internet]. 2008 [cited 2016 Feb 25]; 12(2):258-64. Available from: http://www.scielo.br/pdf/ean/v12n2/v12n2a09
26.Vieira M, Padilha MICS. O HIV e o trabalhador de enfermagem frente ao acidente com material perfurocortante. Rev Esc Enferm USP. [Internet]. 2008 [cited 2013 June 25]; 42(4): 804-810. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0080-62342008000400026&lng=en . http://dx.doi.org/10.1590/S0080-62342008000400026
27. Ministério do Trabalho e Emprego(Br). Portaria n. 485, de 11 de novembro de 2005. Aprova a norma regulamentadora 32 (Segurança e Saúde no Trabalho em Estabelecimentos de Saúde) [legislação na Internet]. Brasília; 2005. [cited 2016 Feb 25]. Available from: http://www.mte.gov.br/legislacao/Portarias/2005/p_20051111_485.pdf
28.Locks L, Lacerda JT, Gomes E, Tine ACPS. Qualidade da higienização das mãos de profissionais atuantes em unidades básicas de saúde. Rev esc enferm USF (Online). [Internet]. 2011 Set [cited 2016 Feb 25]; 32(3): 569-75. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1983-14472011000300019&lng=en . http://dx.doi.org/10.1590/S1983-14472011000300019
29. Ribeiro RP, Camargo EMOA, Vianna LAC. Avaliação da temperatura nos centros de materiais esterilizados. Cogitare Enferm. [Internet]. 2008 [cited 2016 Feb 25]; 13(2)268-74. Available from: http://dx.doi.org/10.5380/ce.v13i2.12502
30. Pires FV, Tipple AFV, Freitas LRD, Souza ACS, Pereira MS. Momentos para higienizar as mãos em Centro de Material e Esterilização. Rev. Bras. Enferm. [Internet]. 2016 [citado em 25 fev 2016]; 69(3): 546-51. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0034-71672016000300546&lng=pt . http://dx.doi.org/10.1590/0034-7167.2016690318i