RESEARCH ARTICLES
Drug interactions in prescriptions for elderly hypertensive patients: prevalence and clinical significance
Natália Balera Ferreira PintoI; Liliana Batista VieiraII; Fernanda Maria Vieira PereiraIII; Adriano Max Moreira ReisIV; Silvia Helena De Bortoli CassianiV
IStudent in the University of São Paulo at Ribeirão Preto, College of Nursing. Ribeirão Preto, São Paulo, Brazil. E-mail: nataliabfp@yahoo.com.br.
IIPharmacist, Doctor in Sciences in the University of São Paulo at Ribeirão Preto, College of Nursing. Ribeirão Preto, São Paulo, Brazil. E-mail: lilianabvieira@yahoo.com.br.
IIINurse, PhD student in the University of São Paulo at Ribeirão Preto, College of Nursing. Ribeirão Preto, São Paulo, Brazil. E-mail: fernanddamaria@hotmail.com.
IVPharmacist, Doctor in Sciences in the University of São Paulo at Ribeirão Preto, College of Nursing. Department of Pharmaceutical Products, Professor, Federal University of Minas Gerais, Pharmacy School. Belo Horizonte, Minas Gerais, Brazil. E-mail: amreis@outlook.com.
VPhD in Nursing, Full professor in the University of São Paulo at Ribeirão Preto, College of Nursing. Regional Advisor on Nursing and Allied Health Personnel, Pan American Health Organization. São Paulo, Brazil. E-mail: sbhcassi@eerp.usp.br.
VIAcknowledgements: Conselho Nacional de Desenvolvimento Científico e Tecnológico and Fundação de Amparo à Pesquisa do Estado de São Paulo.
DOI: http://dx.doi.org/10.12957/reuerj.2014.7111
ABSTRACT: This study examined the prevalence and clinical significance of drug interactions identified in prescriptions of elderly hypertensive patients. This descriptive study was conducted at the Dom Mielle Primary Health Care Unit in Ribeirão Preto, São Paulo State, from July to December 2011. Interactions were identified using Drug-Reax® Micromedex software and classified by severity, time of onset and documentation. SAS®9.0 software was used for descriptive statistics. Of the 40 patients studied, 65.0% were women and the median age was 71.0 (SD = 5.9) years. The average number of drugs prescribed per patient was 7.5 (SD = 2.8); all patients had at least one drug interaction. Of the 169 interactions identified, 17.1% were severe. The occurrence of drug interactions was high in the patients studied. Healthcare teams must identify drug interactions of clinical significance and implement measures to prevent and monitor them.
Keywords: drug interactions; older adults; hypertension; primary health care.
INTRODUCTION
As people age, they end up developing chronic diseases such as hypertension, diabetes, hypercholesterolemia and cardiovascular diseases, and start using a great number of drugs. Those can bring benefits when correctly used by the patient, but can also be harmful if not properly used.
This study was part of doctoral project developed in the University of São Paulo at Ribeirão Preto, College of Nursing, which evaluated the adherence to the medication therapy after an intervention in the medication dispensing. During the pharmacotherapeutic follow-up of the patients of this project, there was the need to study the interactions among the medications used by these participants in order to ensure the rational use.
The use of several drugs may cause interactions among them, which can improve or harm the pharmacological action, bringing serious consequences to the patient. In order to avoid serious consequences, health professionals such as doctors, pharmacists and nurses must know the types of interactions that can occur among the drugs prescribed in the basic-health units and the monitoring processes, so that both the safety of the medication therapy and the patient´s health are ensured.
In this context, aiming at contributing to the understanding of the subject, the present study had as its goal (from the medical prescriptions of elderly hypertensive patients at a basic health unit in the state of São Paulo) to analyze the prevalence of potential drug interactions of the drug-drug type and their clinical importance.
THEORETICAL REFERENCIAL
The proportion of elders has been increasing in the Brazilian population, and that aging is explained by the continuation of the fecundity decline process and, at the same time, the increase in life expectancy, both for men and women1. The elderly population, due to cognitive and physical alterations associated to aging, has an increased risk to problems of medication adherence and may present difficulties in the use of their medication. Polypharmacy and the therapeutics inadequacy, besides increasing the demand for health services and the risk of adverse reactions and medication interactions, favor hospital commitment or contribute to extend its duration2, causing health costs to soar3-5.
The increase in the elderly population contributes to the high prevalence of a number of varied diseases whose treatments, most of the times, include pharmacological resources4. Among the various chronic diseases that the elders present is hypertension, which also demands high medical and socio-economical costs, and mainly stems from complications such as: cerebrovascular disease, coronary heart disease, heart failure, chronic renal failure and vascular diseases of the limbs and peripheral arterial disease6.
The severity, prevalence and possible consequences of the drug interactions are related to such variables as clinical conditions of the individuals and number and characteristics of the drugs7,8. The elderly population is subject to a significant prevalence of interactions that demand the analysis of the possible use of therapeutic alternatives, whether pharmacological or not, or of alterations either in the doses or in administration route of the drugs involved4.
This way, it becomes important to identify the potential interactions in the treatment of hypertension and other chronic diseases, and thus perform the suitable pharmacological management in order to avoid serious side effects. The health professionals must pay close attention to the interactions among drugs and must be able to describe the result of potential interactions and suggest appropriate interventions8,9.
METHODOLOGY
A descriptive, observational and cross-sectional study conducted in the Dom Mielle Basic Health Unit located in the city of Ribeirão Preto, in the state of São Paulo, from July to December 2011. From the health database of the city´s software, a spreadsheet was generated with the Excel® software, containing the list of elderly patients aging 60 or more.
The inclusion criteria for the study were: patients aging 60 years old or more; continuous use of four or more different drugs, being at least one anti-hypertensive; hypertensive patients with systolic blood pressure ≥ 130mmHg; users of the pharmacy service of the Basic Health Unit and under medical follow-up. We excluded patients who were users of insulin and those incapable of administering their own medication, dependent on other people to do so. The inclusion criteria of four or more drugs was meant to ensure the participation of elders with moderate or high pharmacotherapy, adopting the cut-offs: moderate of 4 to 5 and high above 510,11.
From the list on the Excel® software, 265 patients were pre-selected. After attempts of phone calls, 28 did not wish to participate; 19 had difficulties in walking; 10 moved out to another area; 01 was living at an old people´s home; 02 had mental disabilities; 09 worked the whole day and would not be able to participate; 04 had passed away; 34 did not show up for the first meeting (three attempts were made); 53 were not found and 105 patients showed up for the first meeting and were received at the Basic Health Unit. In this meeting, the blood pressure measurement was performed three times: the first measurement was ignored and the means of the last two was calculated. An Omron HEM-742 digital device was employed, which was calibrated and validated. Out of these 105 patients, 59 presented systolic blood pressure < 130mmHg, 06 did not want to participate and 40 did not meet the inclusion criteria. Thus, the study population was composed by 40 patients.
We had two meetings with each patient: the first one in the pre-selection and the second one for the data collection. The interviews took place in the Basic Health Unit, in a reserved room, with an average duration of 30 minutes. An instrument composed by socio-demographic information was applied: the hypertension diagnosis time and the comorbidities presented. The patients´ prescriptions were also transcribed in an instrument containing the prescription date, the patient´s identification number, the names of the drugs, the dose, the frequency, the timetable and the time of use. A pilot study with 5 patients randomly selected from the Basic Health Unit was performed aiming at testing the data collection instruments.
The drug interactions, of the drug-drug kind, present in the elders´ prescriptions were identified by means of the Drug-Reax® software from the Micromedex database of Truven Health Analytics12. The interactions were classified regarding the severity, documentation and time of onset, using the Drug-Reax® specifications:
The drugs involved in the drug interactions were analyzed regarding their adequacy to the elders, according to Beers´ criterion13, once that it is the most widely disclosed in the literature and also because it lists all the drugs that must be avoided by the elders due to their posing a high risk13.
The database was structured on the Excel spreadsheet (2007 version). Double-typing and data validation were performed for the identification of possible errors. The statistical analysis was performed by means of the Statistical Analysis Software (SAS) Version 9.0, comprehending the descriptive statistical analysis and Pearson´s correlation test. Values of p ≤ 0.05 were considered significant.
The research project was authorized by Secretary of Health of Ribeirão Preto and approved by the Ethics Committee of University of São Paulo at Ribeirão Preto, College of Nursing, under the protocol number 00712212.6.0000.5393. The research participants´ authorization was requested with the signing of the Informed Consent form.
RESULTS AND DISCUSSION
The study population was composed by 40 elders, being 26 (65.0%) persons of the female sex and 14 (35.0%) of the male sex. The average age was 71.0 years old (SD=5.9). Concerning the educational background, 9 (22.5%) were illiterate, 22 (55.0%) answered they had studied between 1 and 4 years, 8 (20.0%) studied between 5 and 11 years and only 1 (2.5%) had college-level education.
The average time of hypertension diagnosis was 17.9 years (SD=9.6) and of the use of medication was 18.3 years (SD=8.9), where 18 (45%) had been using medication for more than 20 years. The means of the number of diagnosis was 2.9 (SD=1.1). Besides hypertension, 17 (42.5%) of the patients presented dyslipidemia and Diabetes Mellitus. The average systolic blood pressure was 150.9 mmHg and diastolic blood pressure was 77.8 mmHg.
Regarding the number of drugs prescribed per patient, the means was 7.5 (SD=2.8), adding up to 299 drugs for the 40 patients of the study. Among the most used drugs, acetylsalicylic acid (ASA) was present in 31 (77.5%) of the medical prescriptions, followed by hydrochlorothiazide - 25 (62.5%), simvastatin - 24 (60.0%), omeprazole - 24 (60.0%), metformin - 22 (55.0%), enalapril - 22 (55.0%), glibenclamide - 11 (27.5%), captopril - 7 (17.5%), among others.
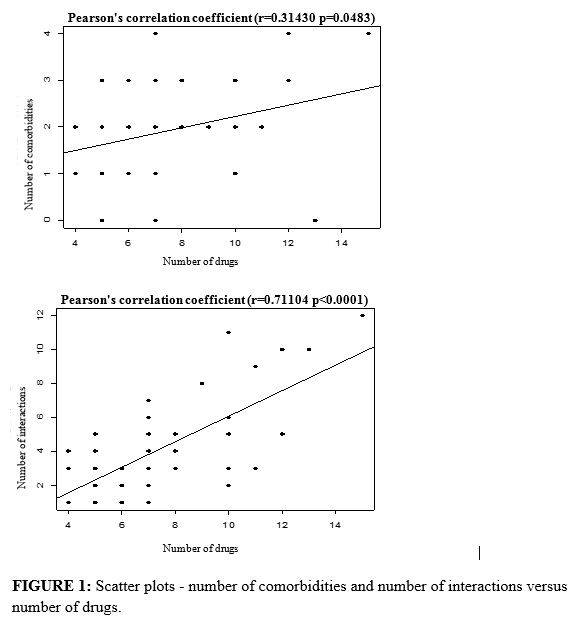
In Pearson´s correlation analysis, the number of drugs presented a statistically significant correlation (p<0.05) with the presence of comorbidities (r=0.314; p=0.0483), and with the drug interactions (r=0.711; p<0.0001), i.e., a directly proportional relation between the number of drugs used by the patients and comorbidities and the number of drug interactions. See Figure 1.

Concerning the drug interactions, the means 4.2 (SD=2.9) was obtained per patient. All the elders included in the study presented at least one interaction and 169 drug interactions were identified. According to the severity, 139 (82.2%) were classified as moderate, 29 (17.2%) were severe and 01 (0.6%) was light, totaling 69 different types. As for the time of onset, 54.7% of the reactions were late and 22.7% were immediate. Regarding the scientific documentation, 60.0% were good and 14.7% were excellent.
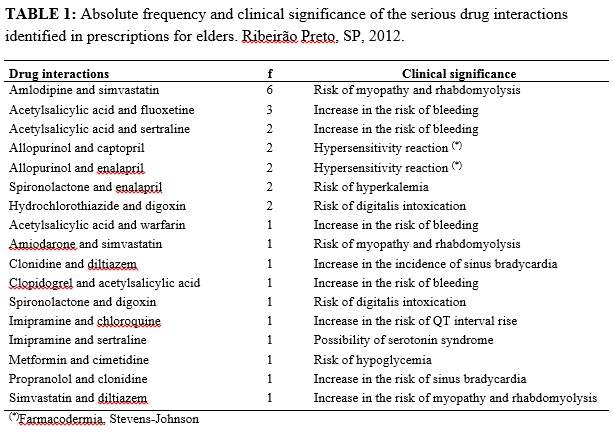
The most frequent serious interaction in this study was the association of the drugs amlodipine and simvastatin, observed in 6 (15.0%) of the medical prescriptions. The most prevalent serious interactions in the study and their clinical implications, according to Drug-Reax®, are presented in Table 1.
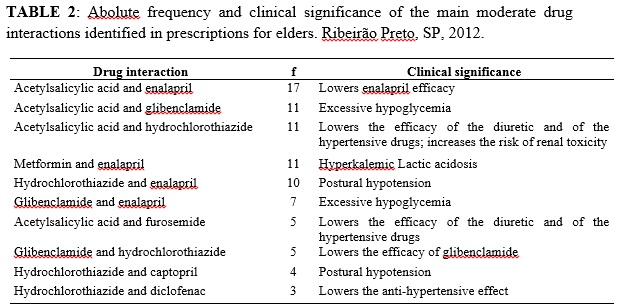
The most prevalent moderate interaction was between ASA and enalapril 17 (42.5%). The main moderate medication interactions identified in the medical prescriptions and their clinical implications, according to Drug-Reax®, are presented in Table 2.
Among the drugs mostly used by the elders, 37 drugs participated in the 69 types of medication interactions identified in the study. The drugs responsible for most interactions were ASA (16 types); enalapril (8); hydrochlorothiazide (9); glibenclamide (6); metformin (7); simvastatin (5); amlodipine (4); captopril (4) and furosemide (4).
Regarding the number of interactions, ASA added up to 64; enalapril 51; hydrochlorothiazide 41; glibenclamide 26; metformin 19; simvastatin 11; amlodipine 9; captopril 10 and furosemide 11. The drugs present in the interactions, considered as inappropriate for elders, according to Beers´ criterion13, were: glibenclamide (26), diclofenac (9), fluoxetine (5), spironolactone (>25mg/day) (5), digoxin (>0.125mg/day) (6), imipramine (2), amiodarone (1), doxazosin (1), fenobarbital (1) and methyldopa (1).
The study demonstrated that the number of drug interactions is directly proportional to the number of drugs and comorbidities, and also evidenced that interaction is a very frequent event in the elders assisted in the primary care included in this study. This age group, habitually, presents several chronic diseases that lead to the phenomenon of polypathology and, consequently, to a high rate of both the use of health services and multiple medication. The medication contributes to the elder´s life quality improvement, but can also cause adverse events that lead to damage, some of them determined by drug interactions. The consumption of multiple medication contributes to the occurrence of undesirable medication interactions7,15-17.
In a quantitative approach, the use of multiple medication is associated to drug interactions in different studies15,17. The population investigated used an average of 7.5 drugs, what allows us to classify that as high polypharmacy. In a study organized in the city of Blumenau, also in the field of the primary health care, the average of drugs used by the elders was 6.6 per elder18.
Several classifications are employed to classify polypharmacy, comprehending lower polypharmacy - use of between 2 and 4 drugs and higher polypharmacy – use of 5 or more drugs. Still, there can be lower polypharmacy – 2 or 3 drugs, moderate – 4 or 5 drugs and high – more than 5; those can also be divided into extracts 1-3, 4-5, 6-8 and more than 9 drugs. The term polypharmacy has also been employed with several connotations, such as the use of inappropriate drugs, occurrence of drug interaction and adverse effect of a drug treated with another drug10,11.
The elders presented an average of 2.9 referred diagnoses and, just like in other studies, the cardiovascular diseases were the ones that mostly incised. Because they are chronic, many of these diseases demand a high cost from the health assistance, and favor the onset of complications with a great interference upon the degree of dependence and quality of life of the people19.
ASA was the mostly used drug in this study, followed by hydrochlorothiazide and simvastatin. Thus, like in an investigation conducted with hypertensive elders in the south of Brazil, ASA, enalapril and hydrochlorothiazide were among the most used drugs and accounted for the main medication interactions of moderate severity18. Corroborating with this finding, literature review pointed out that ASA has been among the main drugs with a potential for drug interaction20.
The medication interactions with ASA are frequently overestimated in most of the studies, because the dose used by the patients is not considered in the studies and by the pieces of software employed in the identification of interactions. The dose is an important factor in the interactions with this drug, because many times it occurs only in doses for use as an analgesic. Among the elders, the high use is associated with the indication as an antiplatelet drug, whose initial dose is normally 100mg. ASA has been used in the prevention of thromboembolism for a long time21; however, the dose that may range from 50 to 325 mg/day22,23 is still nowadays a target for researches and discussions, due to the risk of side effects in higher doses or the loss of therapeutic efficacy in lower doses21.
The interactions of ASA are dose-dependent with the following drugs: enalapril, hydrochlorothiazide, furosemide, captopril, spironolactone, diltiazem, verapamil and indapamide12. Such interactions occur in analgesic doses with clinical significance because they comprise the drug therapeutic response with the cardiovascular action. In this sense, it is important to warn the elders about the risks of the self-medication with ASA.
The interactions with ASA and enalapril, detected in this study, have the potential to cause excessive, showing the relevance of this interaction and the necessity to monitor these patients or, when available, substitute the sulfonylurea for another one more suitable to the elderly12. Glibenclamide is an inappropriate drug to elders, according to Beers´ criteria, because it poses a high risk of producing prolonged acute hypoglycemia in the elder13.
The serious drug interactions, detected in the present investigation, may comprise the safety of the patient´s pharmacotherapy because they potentially increase the risk of bleeding, cardiotoxicity (bradycardia, digitalis intoxication, QT interval alteration) and reactions of hypersensitivity, causing the elders to have the chance of presenting adverse reactions that may comprise their functionality and quality of life12.
Inadequate drugs to the elders, such as fluoxetine, imipramine, clonidine, and amiodarone participate of severe interactions, showing the need to re-evaluate the indication, analyze the dose used (keep digoxin < 0.125mg), consider the therapeutic alternative and identify monitoring strategies when the drug does not present such an alternative, as it frequently happens with the use of amiodarone12. Such monitoring strategies are applied to all drug interactions.
Due to the alterations of e and senility, the elders present physiological alterations, especially those related to the renal functioning, which may comprise the and the pharmacodynamics of the drugs. The interactions of + diclofenac and captopril + diclofenac, detected in this study, besides comprising the anti-hypertensive effect, present the potential to induce renal dysfunction in the elderly. In addition, diclofenac is an inadequate drug to the elderly because it also increases the risk of gastrointestinal bleeding12. Beers´ criteria13 point out that elders above 75 years old that used corticosteroids, antiplatelet drugs and along with non-steroidal anti-inflammatories presented risks of gastrointestinal bleeding. The criteria also point out that the risks increase with the prolonged use.
Amlodipine and simvastatin and amiodarone + simvastatin, when administered together, present serious interactions, what may cause an increase in the exposure to simvastatin and an increase in the risk of myopathy, including rhabdomyolysis. If the concomitant use of such drugs is necessary, the dose of simvastatin must not exceed 20 mg/day, according to the warning published by the Food and Drug Administration (FDA)12,24.
In the practice, many times, the drug interaction is determined by the patients´ clinical condition. In the case of the elders, due to the complexity of the pharmacotherapy and multiple pathologies, the concomitant use of the drugs is usually indicated; however, in order to ensure safe and effective pharmacotherapy, the health team must know how to identify the interactions and recognize the monitoring strategies.
It is worth mentioning that care is the structural element in the practice of nursing. Therefore, it is necessary to identify, study, know and analyze the practices of care that are used by nurses in the field of the primary health care27. The nurse can act and contribute to the improvement of the quality of the services delivered in this area.
Among the limitations of this investigation, we point out the design of the method used for the analysis of the interactions, which must be considered in the interpretation of the data. The software-based identification of the drug interactions detects only the potential interactions, what does not mean that the possible adverse events manifest clinically in all the patients of a potential drug-drug interaction. The software is an important instrument to verify potential drug interactions, but it generally produces a high signal level that can indicate higher interaction prevalence25. However, a differential aspect of this investigation that contributes to the validation of the results obtained is the fact that we have employed a piece of software that presents adequate sensitivity and specificity for the identification of potential drug interactions25,26.
The identification of the interactions was made without observing the dose of the drug and the treatment duration. Thus, it is likely that the prevalence has been overestimated because some interactions can be dose-dependent and the inhibition processes and enzymatic induction are time-dependent. Such enzymatic processes are determinant in the pharmacokinetic interactions, involving the metabolism of the drugs. Another limitation was the convenience sample, comprehending only one basic health unit, an aspect that limits the generalization of the results.
Therefore, new studies may be conducted comprehending a larger population, observing the dose of the drug and the treatment duration, in order to widen the knowledge of the health professionals, ensuring the patient´s safety.
CONCLUSION
The number of drug interactions in the patients´ prescriptions of this study was directly proportional to the number of drugs and comorbidities. The occurrence of drug-drug interactions was high in the patients investigated, comprehending interactions of clinical significance. The interactions detected may present and induce adverse events such as: hypoglycemia, cardiotoxicity, inefficiency of the anti-hypertensive therapy and bleedings that comprise the safety of the elder´s pharmacotherapy, significantly interfering in the functionality and health-related quality of life.
This way, the study contributed to the knowledge of the profile of the drug interactions in hypertensive elders, becoming an important tool for the planning of actions targeting the improvement of the safety of the chronically hypertensive patient making use of multiple medication in the primary health treatment.
REFERENCES
1.Instituto Brasileiro de Geografia e Estatística. Censo Demográfico 2010: Características gerais da população, religião e pessoas com deficiência [Internet]. Rio de Janeiro; 2010 [citado em set 2014]. Disponível em: ftp://ftp.ibge.gov.br/Censos/Censo_Demografico_2010/Caracteristicas_Gerais_Religiao_Deficiencia/caracteristicas_religiao_deficiencia.pdf.
2.Nóbrega OT, Karnikowski MGO. A terapia medicamentosa no idoso: cuidados na medicação. Cien Saude Colet [Internet]. 2005 [citado em 05 set 2014]; 10: 309-13. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1413-81232005000200008&lng=en.
3.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005; 43: 521-30.
4.Costa SC, Pedroso ERP. A prescrição de medicamentos para idosos internados em serviço de clínica médica: atualização. Rev Med Minas Gerais. 2011; 21: 201-14.
5.Santos TRA, Lima DM, Nakatani AYK, Pereira LV, Leal GS, Amaral RG. Consumo de medicamentos por idosos, Goiânia, Brasil. Rev Saude Publica. 2013, 47: 94-103.
6.Sociedade Brasileira de Hipertensão. Diretrizes Brasileiras de Hipertensão Arterial VI. Rev Hipertensão [Internet]. 2010 [citado em 05 abr 2014]. Disponível em: http://www.sbh.org.br/pdf/diretrizes_final.pdf.
7.Secoli SR. Polifarmácia: interações e reações adversas no uso de medicamentos por idosos. Rev Bras Enferm. 2010; 63: 136-40.
8.Amaral DMD, Perassolo MS. Possíveis interações medicamentosas entre os antihipertensivos e antidiabéticos em participantes do Grupo HIPERDIA de Parobé, RS (Uma análise teórica). Rev Ciênc Farm Básica Apl. 2012; 33: 99-105.
9.Carreira CFS, Barrêto VFT, Moura APG, Silva PRJ, Teixeira NAM, Canavieiras AS. Interações medicamentosas: um relato de caso sobre a avaliação e intervenção farmacêutica. In: 11. Encontro de Iniciação à Docência; 2008; João Pessoa: UFPB, 2008.
10.Salazar JA, Poon I, Nair M. Clinical consequences of polypharmacy in elderly: expect the unexpected, think the unthinkable. Expert Opinion Drug Safety. 2007; 6: 695-704.
11.Silva AL. Estudo de utilização de medicamentos por idosos brasileiros [dissertação de mestrado]. Belo Horizonte (MG): Faculdade de Farmácia da Universidade Federal de Minas Gerais; 2009.
12.Micromedex Healthcare Series [Internet]. Truven Health Analytics. 2012 [citado em 05 set 2014]. Available in: http://www-micromedexsolutions-com.ez67.periodicos.capes.gov.br/micromedex2/librarian/.
13.American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012; 60: 616-31.
14.Soares MA, Fernandez-Llimos F, Cabrita J , Morais J. Tools to evaluate potentially inappropriate prescription in the elderly: a systematic review. Acta Med Port. 2011; 24: 775-84.
15.Obreli Neto PR, Nobili A, de Lyra DPJ, Pilger D, Guidoni CM, Oliveira Baldoni A, et al. Incidence and predictors of adverse drug reactions caused by drug-drug interactions in elderly outpatients: a prospective cohort study. J Pharm Pharmaceut Sci. 2012; 15: 332-43.
16.Passarelli MCG, Jacob Filho W. Reações adversas a medicamentos em idosos: como prevê-las? Einstein. 2007; 5: 246-51.
17.Lea M, Rognan SE, Koristovic R, Wyller TB, Molden E. Severity and Management of Drug-Drug Interactions in Acute Geriatric Patients. Drugs Aging. 2013; 30:721-7.
18.Codagnone Neto V, Garcia VP, Santa Helena ET. Possible pharmacological interactions in hypertensive and/or diabetic elderly in family health units at Blumenau (SC). Braz J Pharm Sci. 2010; 46: 795-804.
19.Marin MJS, Cecílio LCO, Perez AEWUF, Santella F, Silva CBA, Filho Gonçalves JR, et al. Caracterização do uso de medicamentos entre idosos de uma unidade do Programa Saúde da Família. Cad Saúde Pública. 2008; 24: 1545-55.
20.Silva LD, Santos MM. Interações medicamentosas em unidade de terapia intensiva: uma revisão que fundamenta o cuidado do enfermeiro. Rev enferm UERJ. 2011; 19: 134-9.
21.Araujo BG, Menezes AC. Dose do AAS como Anti-agregante plaquetário. In: Souza PM, Araujo BG, Silva LP, organizadores. Farmacologia clínica: textos informativos. Brasília (DF): Universidade de Brasilia; 2012. p. 88-90.
22.Ansara AJ, Nisly SA, Arif SA, Koehler JM, Nordmeyer ST. Aspirin dosing for the prevention and treatment of ischemic stroke: an indication-specific review of the literature. Ann Pharmacother. 2010; 44: 851-62.
23.Ministério da Saúde (Br). Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Assistência Farmacêutica e Insumos Farmacêuticos. Formulário Terapêutico Nacional: Rename 2008. Brasília (DF): Editora MS; 2008.
24.Food Drug Administration. Drug safety communication: new restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury, 2011. [cited 15 Set 2014]. Avaible : http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm.
25.Reis AM, Cassiani SH. Evaluation of three brands of drug interaction software for use in intensive care units. Pharm World Sci. 2010; 32: 822-8.
26.Vonbach P, Dubied A, Krähenbühl S, Beer JH. Evaluation of frequently used drug interaction screening programs. Pharm World Sci. 2008; 30: 367-74.
27.Ferreira VA, Acioli S. O cuidado na prática do enfermeiro no campo da atenção primária em saúde: produção científica. Rev enferm UERJ. 2009; 17: 506-9.