ORIGINAL RESEARCH
Risk of exposure to biological material at primary health care facilities
Keyti Cristine Alves Damas RezendeI; Anaclara Ferreira Veiga
TippleII; Adenícia Custódia Silva e SouzaIII;
Karina Machado SiqueiraIV; Sergiane Bisinoto Alves V; Thaís de Arvelos SalgadoVI
I
Nurse. Master's Degree in Nursing from the Federal University of Goiás.
Nurse of Maternal-Infantile Hospital. Goiania, Goias, Brazil. E-mail: keytidamas@hotmail.com
II
Nurse. PhD Degree in Nursing. Professor at the Federal University of Goias.
Goiania, Goias, Brazil. E-mail: anaclara.fen@gmail.com
III
Nurse, PhD Degree in Nursing. Professor at the Catholic University of
Goias, Goiania, Goias, Brazil. Email: adeniciafen@gmail.com
IV
Nurse. PhD Degree in Nursing. Professor at the Federal University of Goias,
College of Nursing. Goiania, Goias, Brazil. E-mail: karinams.fen@gmail.com
V
Nurse. PhD Degree in Nursing. Nurse at the Hospital das Clínicas of the
Federal University of Goias. Brazil. E-mail:
sergianebisinoto@yahoo.com.br
VI
Nurse. Master's Degree and Doctoral Candidate at the Federal University of
Goias. Brazil. E-mail: thais.arvelos@hotmail.com
DOI:
http://dx.doi.org/10.12957/reuerj.2016.6442
ABSTRACT
Objective:
to identify modes of exposure to biological material involving nursing
professionals at Primary Care Centers in a Health District of Goiânia,
Goiás. Method: once ethical requirements were met, data
were obtained by questionnaire and direct observation, and recorded on a
check list. From January to May 2010, 149 procedures were observed:
neonatal and antenatal screening, Papanicolaou test, immunization and
dressings. Results: risk of exposure to biological
material was found during sharps handling, possible contact with blood,
secretions and immunobiologics, aerosol formation, proximity between an
accessed limb and nurse's face, and agitation and/or unexpected reaction
from user. Conclusion: there is risk of exposure to
biological material in procedures performed by primary health care nurses
and that risk is heightened by non-observance of standard precautions.
There is a need for infection control committees in the health district, to
give guidance and supervision on safe procedures.
Keywords: Risk management; primary health care; protective devices; universal
precautions.
INTRODUCTION
Healthcare-related infections (HCRI), including those identified in the
primary healthcare (PHC) services, have represented a severe problem and
various effects in human health context. Under the perspective of worker's
health, the biological risk (BR) focuses on the likelihood of occupational
exposure to biological agents1, arising from the presence of
pathogens at the workplace. Such an exposure is divided into two
categories: exposition with deliberate intention, from the labor activity
as a result from directly non-deliberately using or handling the biological
agent stemming from the labor activity without the direct handling of this
agent. Knowing risks genesis, the protective measures to be implemented can
achieve higher electivity1.
From user perspectives, HCRI represent one of the key indicators for
quality of care, because HCRI are among the leading causes for morbidity
and mortality and, consequently, cost elevation for the treatment2. The user, when looking for the health service, is vulnerable
to developing infections by microorganisms from exogenous and endogenous
sources3-5.
PHC represents, today, one of the gateways for the Unified Health System
(SUS). Thus, the for Integral Healthcare Centers (IHC), which are
non-hospital units for urgency and emergency care, and the Basic Health
Units (UBS) offer broad service with medium and low complexity. In these
units, nursing professionals perform a range of procedures that expose them
to occupational hazards, among which the BR stands out.
Accidents involving biological material show risk for infection and may
cause emotional and psychological impact on the professional, in addition
to the financial costs on the healthcare system with post-exposure
prophylaxis and worker's follow-up6.
Thus, it is believed that BR characterization, involved in providing cares
in PHC, can offer subsidies for managers and employees in developing and
implementing a security policy in that context. Thus, this study aimed to
identify exposure modes to biological material found in the activities
carried out by nursing professionals who work in PHC units of a Health
District at the city of Goiania, Goias, Brazil.
LITERATURE REVIEW
PHC is configured as a communication hub with all Healthcare Network and
assumes a central role in ensuring access for the population for the
services in this network. In this context, special mention deserves to be
directed to Family Health Strategy (FHS), adopted by the Ministry of Health
in 1994, as an instrument for developing PHC in Brazil, by means of the
Family Basic Healthcare Units (FBHCU). PHC qualification and consolidation
appears as an expansion strategy, through the reorientation of work
processes, increasing the efficaciousness and impact on people's health
situation and collectivities7.
Significant numbers of health professionals work in the PHC, including in
FHS, however, the discussions geared specifically to the risks to which
workers are exposed in this scenario are meager. The literature on BR and
adopting standard precaution (SP) measures in the hospital area is vast,
but it's only recently that this subject has been addressed in the PHC
context8-12.
Given this, it is important to stress out that the employment of safe
practices and the use of adequate protective equipment can significantly
reduce the risk for occupational accidents, including those related to
exposure to biological agents. Biosecurity, in its broader perspective,
aims as central objective to provide the professionals and institutions
with tools that allow for developing safe activities, whether for health
protection or environmental protection13.
In 2007, the Centers of Disease Control and Prevention (CDC)
published the new practices for preventing and controlling infection,
reaffirming the fundamental elements for preventing transmission of
infectious agents in healthcare services and bringing updates in relation
to the previous publication, which had been disclosed in 1996 and disserted
on precautions related to hospital services14. It is important
to note that among the justifications for revising this material is the
recognition that healthcare is provided in places that differ from hospital
ambience, among which we may refer clinics, long permanence institutions
and the domicile, where the professionals perform both administrative
activities as well as social ones, such as they have been identified in the
investigation15.
The complications generated by oversights related to infection control in
the procedures performed by professionals in the PHC, include:
broncho-aspirations, appearance of infected pressure ulcers, infections
related to invasive procedures such as vascular catheters, vascular probes,
among others 16. In this sense, it should be noted
that infection control should be part of the quality indicators to be
followed in the PHC, including homecare, because many complications and
user-related inter-occurrences may result from lack of control on the
procedures involved in the care.
METHODOLOGY
Cross-sectional, descriptive study, with a quantitative approach, conducted
with nursing professionals (nurses, nursing technicians and assistants)
working in two BHU, nine FBHCU and three IHCs, belonging to a Health
District of the municipality of Goiania-GO.
Considering the limited literature on the subject, some professional
nurses, operating in the infection control areas and/or PHC were invited to
discuss the study's e proposal. Eight professionals took part in this
initial step which purposed to list which procedures developed in the PHC
offered to BR to professional and user.
Group consensus was to include procedures involving handling sharps and/or
contaminated items, contacts with sterile mucosal sites that offer the
possibility to exposure to blood and secretions. Five procedure types were
elected: newborn screening tests and pre-natal screenings, Pap smear,
vaccinations and dressings. The exposure modes to the biological material
were extracted from the observation made when performing the elected
procedures.
For data collection, a check list specific for each procedure completed
during the direct non-participant observation was prepared. Each check list
included the steps on the respective procedures and preventive measures
provided for the safety of professionals and users. A questionnaire with
closed and open questions for characterizing the professional who performed
the procedure was observed and the qualification for the work was applied
after this step.
Data collection instruments were evaluated by three experts in infection
control for checking their operation capacity. In addition, a pilot test
was conducted in PHC service of another municipality. After each unit
coordinator's consent, upon presenting the Ethics Committee's approval and
authorization by the Municipal Health Secretariat of Goiania, data
collection began, which occurred in the period from January to May, 2010.
We standardized for each unit, 20 observation hours, divided into shifts
according to the schedule and work demand, totaling approximately 280
hours.
The project was approved by the Ethics Committee in Human and Animal
Research of the Hospital das Clínicas of the Federal University of Goias -
Protocol No. 029/09, according to the Brazilian research recommendations
for research with human beings 17. At the end of the observation
period, the subjects were informed about the study objectives and after
having access to the records arising from the observation, those who
agreed, signed the Free and Clarified Consent Form (FCCF) and responded to
the questionnaire.
We used the Statistical Package for Social Sciences (SPSS)
software, version 16.0 for Windows for descriptive analysis, using absolute
and percentage frequency.
RESULTS AND DISCUSSION
149 comments were made regarding 77 vaccinations, 28 dressings, 28 Pap
smears, 11 heel prick tests, and 9 prenatal screenings.
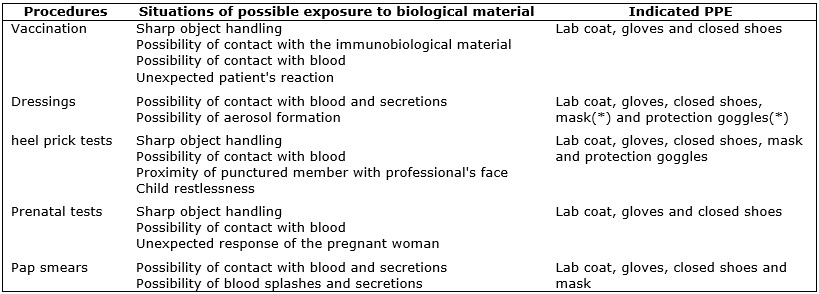
When looking at the exposure modes to biological material in each procedure
and the indicated respective personal protective equipment (PPE), it was
found that all observation-elected procedures showed the possibility for
contact with blood and, in three of the five types of procedures, sharps
were used. It has also been observed the possibility for forming splashes
and aerosols, accomplishing the dressings; risk related to the level of
shaking the child and proximity of punctured member with the professional's
face, upon collecting material for the heel prick test and exposure risk
due to unexpected response from the pregnant woman in the fingerstick for
prenatal screenings, as shown in Figure 1.
(*) The choice of appropriate PPE depends on the dressing evaluation and
the planned exposure level.
FIGURE 1:
Procedures and situations of possible exposure to biological material and
indicated respective personal protective equipment (PPE). Goiânia, Goiás,
Brazil, 2010.
Study performed in FBHCU in Sao Carlos-SP, Brazil, 238 procedures were
observed involving potential contact risk with biological material, and
more than 90% involved the use of needles 11. All actions
involving interaction between user and healthcare professional, known to be
related to the risk of exposure to biological material, must be surrounded
by prudence and require precautionary measures to minimize contamination
chance.
Using PPE is considered a SP, so that it is recommended in care for all
users, regardless of presumed infection state, in situations where there is
a risk for contact with blood, body fluids, organic-origin secretions and
excretions 14. Indicating a certain PPE is also based on the
presumed exposure risk for professionals and users in a procedure.
The gloves are designed to prevent hand contamination of the professionals,
but also protect users from exposure to microorganisms found in outer
sources. Short coats are used to protect your arms and body exposed areas,
also avoiding a possible contamination of the own clothes. Masks and
goggles should be used given the possibility for contact with respiratory
secretions a blood d aerosols or body fluids13. The
Regulatory Norm - NR 321 includes closed footwear as mandatory
PPE for healthcare professionals.
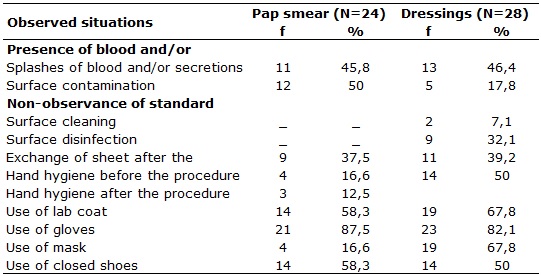
In relation to situations where the exposure to biological material was
found during the Pap smears performed by nurses and dressings made by
technicians in nursing, in 11 (45.8%) and 13 (46.4%), respectively, there
was noted the risk for blood splashes and secretions, situations in which
all professionals had possibility for ocular mucosa exposure as shown in
Table 1, where the goggles do not appear, because no professional has used
them.
TABLE 1:
Situations related with exposure risk to biological material for bloodspot
tests and dressings in basic healthcare units of a health district.
Goiânia, Goiás, 2010.
It is noteworthy that there are evidences where the eyes rank second as
most affected body region during an accident18,19.
Exposure risk for oral mucosa was also observed and adherence to masks in
Pap smears and dressings was 4 (16.6%) and 19 (67.8%), respectively. In
fact, for all the Pap smears and dressings, there were 100.0% adherence to
the recommended PPE, which allows to infer that there occurred potentiation
for the exposure risk to biological material for the workers and users.
Visible surface contamination was observed in 12 (50.0%) Pap smears and 5
(17.8%) in dressings, however, decontamination cares for these surfaces
were done in smaller periodicities. Just after 9 (32.1%) dressings, one
conducted friction with 70% alcohol with no prior cleaning. A procedure
considered to be inappropriate, once that 1%sodium hypochlorite germicides
and 70% alcohol, available for surface disinfection in the units, have low
action under the presence of organic matter20,21.
It was observed that both for the Pap smear, as well as for dressings,
there was no recommended adhesion to SP. It is interesting to stress out
that the professional technicians in nursing had demonstrated greater care
in minimizing the BR involved in accomplishing the dressings, when compared
with the nurses, when accomplishing the Pap smears. Situation that
compromises the autonomy being necessary for the nurses to guide, supervise
and encourage adherence to SP on the part of their team, functions expected
from this professional22-24, in addition to encouraging a
patient safety culture25,26.
Sheet exchange did not occur during the Pap smears. Sheets were overlaid
with Kraft paper, exchanged for each user. Although a little uncomfortable,
this paper fulfills the function of protecting the gynecological litter
surface from splashes and secretions. In carrying out dressings, one just
proceeded to exchange sheets in the presence of visible filthiness.
In addition to environmental contamination issue, the low hygiene levels on
the hands for the Pap steams and dressings, both before the procedures, 4
(16.6%) and 14 (50.0%), respectively, as well as after, what occurred in
only 3 (12.0%) of tests and after dressings there was no adherence. It is
noteworthy that all sites intended for implementing the procedures had sink
with manually-operated tap, paper towels, and liquid soap and that in half
of these sites, the 70% alcohol was available. Still, at most
opportunities, summing up the moments before and after, nurses and
technicians in nursing did not accomplish their hand hygiene, disregarding
the importance on this simple and low-cost practice22,27,28.
The low adhesion of health professionals to hand hygiene is repeatedly
documented28-32. The non-observance on this measure exposes
professional and users for exposure risk to biological material.
Data from this study allow us to infer that the professionals are replacing
the hand hygiene by using gloves, which had adherence in accomplishing 21
(87.5%) Pap smears and 23 (82.1%) dressings. However, this practice
consolidates a misconception, because using gloves does not replace hand
hygiene and it must occur before and after using this PPE1.
In situations related to exposure risk to biological material during
vaccinations, heel prick tests and prenatal screenings, one observed the
directing of sharps for the professional's hand that was acting, as shown
in Table 2.
TABLE 2:
Situations that configured exposure risk to biological material in
procedures with the use of sharp articles, in basic healthcare units of a
health district. Goiânia, Goiás, 2010.
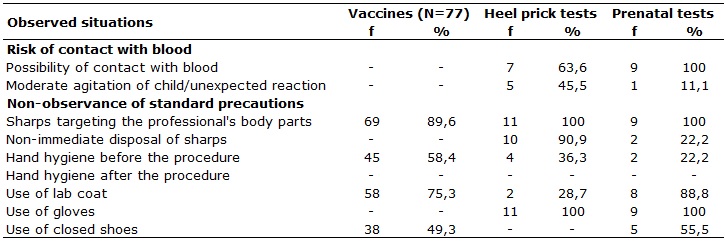
It was found that in all heel prick tests and prenatal screenings, lancet
targeting occurred towards the healthcare professional's hand. In these
procedures, involving fingerstick, or heel puncture, in order to obtain
blood sample for examinations, there is always the direction of the lancet
or needle towards the professional's fingers, since they are required to
firmly hold the place, pressing it with their fingers in order to proceed
to puncture. It is necessary to consider that the risk observed in this
context takes place on the occurrence of accident before the puncture,
which would not be accident with biological material, since that it would
anticipate the contact with the user. However, the physical injury appears
as a gateway to biological agents.
We still emphasize that during fingerstick or heel puncture, the user can
become affected by the pain and collapse the hand or foot. In this case,
there is the possibility of occurring accidents with biological material,
because the professional may cause an accident with the already-used
lancet.
It is noteworthy that, with the mandatory use of safety devices,
established in Brazil by Decree No. 1748 of the Ministry of Labor and
Employment 33, one expects a positive impact on workers' safety,
but that cannot yet be measured in the described situations, using the
lancet with a security device would diminish this risk, offering more
security to the professional and the user.
While conducting vaccinations, it was also noted that at every opportunity
the needle pointed towards the professional hand, because it holds the
member where injectable vaccine will be applied. In this case, the risk for
accidental needlestick injury is potentiated against unexpected user
reactions, especially children.
Study in public health units in Ribeirão Preto - SP, found that among 155
work accidents, 40% involved worker's exposure to potentially contaminated
material, which occurred predominantly with needles in the presence of
blood 8, a reality similar to hospital ambience 34.
The non-immediate discard of sharps occurred while accomplishing 2 (22.2%)
prenatal screenings and in 10 (90.9%) heel prick tests. On these occasions,
after heel puncturing, the used lancet has been deposited on a kidney-type
vat, along with the cotton. In one heel prick tests (9.0%), it was observed
that the amount of blood which flowed was insufficient requiring a new
puncture, which was performed with the same lancet. This procedure has
exposed the newborn at risk of contamination, since that the same lancet
was used, previously deposited in a non-sterile vat. In this situation
there also was a risk for exposure to the professional, both by contact
with blood-stained cotton, as well as with the already-used lancet.
Opposing this practical, one observed that upon vaccination the sharp was
an immediately discarded.
It is important to stress out that, many times, PHC units do not count on
the necessary infrastructure for discarding, and preserving health service
wastes, what potentiate the BR for the involved professionals 35
. Within the hospital there are evidences that accidents occur more often
after use and before disposal of a sharp 36,37.
It is stressed out that all the rooms, where procedures were performed
involving the handling of sharps, had rigid container, resistant to
puncture, rupture and leak, with lid and properly labeled, suitable for
packaging these materials, as recommended by the Brazilian legislation 38,39. It was noted, however, that sharp collectors were not
prepared in appropriate brackets and were exposed to moisture, accessible
to children and distant from the procedure site.
There was no possibility for contact with blood in 7 (63.6%) heel prick
tests. On these occasions, the amount of blood which flowed from the
puncture was extensive, soaking the cotton ball with which the professional
pressured the site. The observed risk was potentiated by the moderate
child's agitation state, present in 5 (45.5%) collections, enabling the
occurrence of blood splashes in oral and ocular mucous membranes, because
in this situation the professional's face is close to the child's heel.
However, safety goggles not even appear in Table 2 as PPE because the
professionals that performed the procedure did not use them.
Upon prenatal screenings, the possibility for contact with blood at every
opportunity is due to the proximity between the professional and the
puncture site, because it is necessary for the professional to press the
user's finger. In this procedure it is also noted that, in one of the
collection opportunities, there was an unexpected reaction of a pregnant
woman to puncturing, (11.1%) increasing the risk of accidents with sharps.
It is stressed out that, unlike what happens with vaccination, held in a
proper room for material's safekeeping and administration, heel prick tests
and prenatal screenings were carried out in different rooms, spaces
available for procedures. These situations hampered the correct disposal of
used lancets in these tests, sometimes requiring their transport, contrary
to the recommendations38.
CONCLUSION
This work made it possible to characterize BR in the activities developed
in the PHC for professionals and users. This characterization is firmed on
the developed procedure nature, being that the exposure was related to the
handling of sharps, possibility for contact with blood, secretions and
immunobiological agents, possibility for aerosol formation, proximity of
punctured member and professional's face, restlessness and/or unexpected
response from the user. Failures related to adherence to SP also
contributed to for the professionals and users to provide greater exposure
to the BR.
The BR is therefore inherent to health practices, regardless of the place
where this practice occurs. Further studies are needed for knowledge on BR
specifics, seeking for applied alternatives and solutions.
As limitation for this study is the absence of data concerning vaccination
situation for the professional participants, which would contribute to
understanding the situation for professional's risk in case of exposure. We
suggest that new researches directed to controlling infections in the PHC
should be carried out so that this theme wins the deserved importance, both
in academic ambience as well as in the professional sphere. Efforts should
be directed to orienting the nursing professionals for reaching a greater
adherence to SP. The nurse, as the leader of this team, should be
encouraged to develop actions with respect to security and the commitment
to minimize the BR inherent to their practice, still acting with
educational actions in this context.
Another important aspect relates to social control possibility to
collaborate for adopting safer practices during healthcare. It is suggested
that the user population for health services be informed on the risk to
which they are exposed, especially in situations where the professionals do
not follow proper preventive measures.
REFERENCES
1. Ministério do Trabalho e Emprego (Br). Portaria nº 485, de 11 de
novembro de 2005. Aprova a norma regulamentadora nº 32 (segurança e saúde
no trabalho em estabelecimentos de saúde). Brasília (DF): Ministério do
Trabalho e Emprego; 2005.
2. Ministério do Trabalho e Emprego (Br). Riscos biológicos. Guia técnico.
Os riscos biológicos no âmbito da norma regulamentadora nº 32. Brasília
(DF): Ministério do Trabalho e Emprego; 2008.
3. Sehulster L, Chinn RY, CDC; HICPAC. Guideline for Environmental
Infection Control in Health-Care Facilities. Recommendations of CDC and the
Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR
Recomm Rep. 2003; 52(RR-10): 1-42.
4. Sornette D, Yukalov VI, Yukalova EP, Henry JY, Schwab D, Cobb JP.
Endogenous versus Exogenous Origins of Diseases. J Biol
Syst. 2009; 17: 225-67.
5. Oliveira AC, Paula AO. Healthcare-associated infections in the context
of patient safety: past, present and future. Rev Min Enferm. 2013; 17:
221-24.
6. Rapparini C, Reinhardt EL. Manual de implementação: programa de
prevenção de acidentes com materiais perfurocortantes em serviços de saúde.
São Paulo: Fundacentro. 2010.
7. Ministério da Saúde (Br). Política Nacional de Atenção Básica. Brasília
(DF): Ministério da Saúde; 2012.
8. Farias SNP, Zeitoune RCG. Riscos no trabalho de enfermagem em um centro
municipal de saúde. Rev enferm UERJ. 2005; 13: 167-74.
9. Chiodi MB, Marziale MHP, Robazzi MLCC. Occupational accidents involving
biological material among public health workers. Rev Latino-Am Enfermagem.
[Scielo-Scientific Electronic Library Online] 2007 [cited on 2016 Jan 5].
15: 632-8. Available from:
http://www.scielo.br/pdf/rlae/v15n4/pt_v15n4a17.pdf
.
10. Nunes MBG, Robazzi MLCC, Terra FS, Mauro MYC, Zeitoune RCG, Secco IAO.
Riscos ocupacionais dos enfermeiros atuantes na atenção à saúde da família.
Rev enferm UERJ. 2010; 18: 204-09.
11. Cardoso ACM, Figueiredo RM. Situações de risco biológico presentes na
assistência de enfermagem nas unidades de saúde da família (USF). Rev
Latino-Am Enfermagem. [Scielo-Scientific Electronic Library Online] 2010
[cited on 2016 Jan 5]. 18: 368-72. Available from:
http://www.scielo.br/pdf/rlae/v18n3/pt_11.pdf
.
12. Figueiredo RM, Maroldi MAC. Internação domiciliar: risco de exposição
biológica para a equipe de saúde. Rev esc enferm USP. [Scielo-Scientific
Electronic Library Online] 2012 [cited on 2016 Jan 5]. 46: 145-150.
Available from:
http://www.scielo.br/scielo.php?pid=S0080-62342012000100020&script=sci_arttext
.
13. Ministério da Saúde (Br). Portaria n.º 2.472, de 31 de agosto de 2010.
Define as terminologias adotadas em legislação nacional, conforme disposto
no Regulamento Sanitário Internacional 2005 (RSI 2005), a relação de
doenças, agravos e eventos em saúde pública de notificação compulsória em
todo o território nacional e estabelecer fluxo, critérios,
responsabilidades e atribuições aos profissionais e serviços de saúde.
Brasília (DF): Ministério da Saúde; 2010.
14. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Health Care Infection
Control Practices Advisory Committee. 2007 Guideline for Isolation
Precautions: Preventing Transmission of Infectious Agents in Health Care
Settings. Am J Infect Control. 2007; 35 (10 Suppl 2): 65-164.
15. Acioli S, Kebian LVA, Faria MGA, Ferraccioli P, Correa VAF. Práticas de
cuidado: o papel do enfermeiro na atenção básica. Rev enferm UERJ. 2014;
22: 637-42.
16. Associação Paulista de Estudos e Controle de Infecção Hospitalar -
APECIH. Prevenção e controle de infecções associadas à assistência médica
extra-hospitalar: ambulatórios, serviços, diagnósticos, assistência
domiciliar e serviços de longa permanência. São Paulo: APECIH; 2004.
17. Ministério da Saúde (Br). Conselho Nacional de Saúde. Resolução
466/2012 – Diretrizes e normas regulamentadoras de pesquisa envolvendo
seres humanos. Brasília (DF): Ministério da Saúde; 2012.
18. Câmara PF, Lira C, Santos Junior BJ, Vilella TAS, Hinrichsen SL.
Investigação de acidentes biológicos entre profissionais da equipe
multidisciplinar de um hospital. Rev enferm UERJ. 2011; [cited on 2016 Jan
5] 19(4):583-6. Available from:
http://www.facenf.uerj.br/v19n4/v19n4a13.pdf
.
19. Marziale MHP, Galon T, Cassiolato FL, Girão FB. Implantação da Norma
Regulamentadora 32 e o controle dos acidentes de trabalho. Acta Paul
Enferm. 2012; [cited on 2016 Jan 5] 25(6): 859-66. Available from:
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-21002012000600006
.
20. Padoveze MC, Graziano KU. Limpeza, Desinfecção e Esterilização:
Aspectos Gerais de artigos em serviços de saúde. São Paulo: APECIH; 2010.
21. Oliveira AC, Damasceno QS. O papel do ambiente hospitalar na
disseminação de bactérias resistentes. Rev Epidemiol Control Infect. 2012;
[cited on 2016 Jan 5] 2(1): 28-31. Available from:
http://online.unisc.br/seer/index.php/epidemiologia/article/view/2625/1838
.
22. Malaguti SE, Hayashida M, Canini SRMS, Gir E. Enfermeiros com cargos de
chefia e medidas preventivas à exposição ocupacional: facilidades e
barreiras. Rev esc Enferm USP. 2008; [cited on 2016 Jan 5] 42: 496-503.
Available from:
http://www.scielo.br/scielo.php?pid=S0080-62342008000300012&script=sci_arttext
.
23. Giarola, LB, Baratieri, T, Costa AM, Bedendo J, Marcon SS, Waidman MAP.
Infecção hospitalar na perspectiva dos profissionais de enfermagem: um
estudo bibliográfico. Cogitare Enfermagem. 2012; [cited on 2016 Jan 5]
17(1): 151-157. Available from:
http://ojs.c3sl.ufpr.br/ojs/index.php/cogitare/article/view/26390/17583
.
24. Dutra GG, Costa MP, Bosenbecker EO. Controle da infecção hospitalar:
função do enfermeiro. J. res.: fundam. care. online. 2015; [cited on 2016
Jan 5] 7(1), 2159-2168. Available from:
http://www.seer.unirio.br/index.php/cuidadofundamental/article/viewFile/3571/pdf_1471
.
25. Ministério da Saúde. Agência Nacional de Vigilância Sanitária.
Segurança do paciente em serviço de saúde: higienização das mãos. Brasília,
2009.
26. Paese F, Dal Sasso GTM. Cultura da segurança do paciente na atenção
primária à saúde. Texto contexto - enferm. 2013; [cited on 2016 Jan 5]
22(2): 302-310. Available from:
http://www.scielo.br/pdf/tce/v22n2/v22n2a05
.
27. Ministério da Saúde (Br). Agência Nacional de Vigilância Sanitária.
Higienização das mãos em serviços de saúde. Brasília (DF): Ministério da
Saúde; 2007.
28. World Health Organization - WHO. The WHO Guidelines on Hand Hygiene in
Health Care (Advanced Draft) – Global Patient Safety Challenge 2005 – 2006:
clean care is safer care. Geneva: WHO Press; 2006.
29. Centers for Disease Control and Prevention - CDC. Guideline for hand
hygiene in health-care settings: Recommendations of the healthcare
infection control practices advisory committee and the
HICPAC/SHEA/APIC/IDSA Hand hygiene task force. MMWR Recomm Rep. 2002;
51(RR-16):1-45.
30. Martins KA, Tipple AFV, Souza ACS, Barreto RASS, Siqueira KM, Barbosa
JM. Adesão às medidas de prevenção e controle de infecção de acesso
vascular periférico pelos profissionais da equipe de enfermagem. Cienc Cuid
Saude. 2008; [cited on 2016 Jan 5] 7: 485-92. Available from:
http://www.periodicos.uem.br/ojs/index.php/CiencCuidSaude/article/viewFile/6634/3908
.
31. Garcia-Zapata MRC, Souza ACS, Guimarães JV, Tipple AFV, Prado MA,
Garcia-Zapata MTA. Standard precautions: knowledge and practice among
nursing and medical students in a teaching hospital in Brazil. Int J Infect
Control. 2010; [cited on 2016 Jan 5] 6: 1-8. Available from:
http://www.ijic.info/article/view/4075/3721
.
32. Oliveira AC, Paula AO. Monitoração da adesão à higienização das mãos:
uma revisão de literatura. Acta Paul Enferm. 2011; [cited on 2016 Jan 5]
24(3): 407-13. Available from:
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-21002011000300016
.
33. Ministério do Trabalho e Emprego. Portaria n° 1.748, de 30 de agosto de
2011. Institui o Plano de Prevenção de Riscos de Acidentes com Materiais
Perfurocortantes e altera a Norma Regulamentadora nº 32, que trata da
segurança e saúde no trabalho em estabelecimentos de saúde. Brasília
(Brasil): Ministério do Trabalho e Emprego; 2011.
34. Velasco AR, Lima FB, Alves EA, Lima ABG, Santos PSSR, Passos JP.
Ocorrência de acidentes de trabalho em saúde com exposição a material
biológico. Rev Enf Profissional. 2014; [cited on 2016 Jan 5] 1(1): 37-49.
Available from:
http://www.scielo.br/pdf/rlae/v21nspe/pt_25.pdf
.
35. Oliveira LL, Souza PM, Clementino FS, Paiva SC, Rocha FDLJ. Resíduos
dos serviços de saúde: desafios e perspectivas na atenção primária. Rev
enferm UERJ. 2014; [cited on 2016 Jan 5] 22(1): 29-34. Available from:
http://www.facenf.uerj.br/v22n1/v22n1a05.pdf
.
36. Rapparini C, Reinhardt EL. Manual de implementação: programa de
prevenção de acidentes com materiais perfurocortantes em serviços de saúde.
São Paulo: Fundacentro; 2010.
37. Julio RS, Filardi MBS, Marziale MHP. Acidentes de trabalho com material
biológico ocorridos em municípios de Minas Gerais Rev Bras Enferm. 2014;
[cited on 2016 Jan 5] 67(1): 119-26. Available from:
http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0034-71672014000100119
.
38. Ministério da Saúde (Br). Agência Nacional de Vigilância Sanitária.
Resolução RDC nº 306, de 07 de dezembro de 2004. Dispõe sobre o Regulamento
Técnico para o gerenciamento de resíduos de serviços de saúde. Brasília
(DF): Ministério da Saúde; 2004.
Ministério do Meio Ambiente (Br). Conselho Nacional do Meio Ambiente.
Resolução 358 de 29 de abril de 2005. Dispõe sobre o tratamento e a
disposição final dos resíduos dos serviços de saúde e dá outras
providências. Brasília (DF): Ministério do Meio Ambiente; 2005.


