RESEARCH ARTICLES
Factors related to the use of flumazenil in hospitalized patients
Valcieny de Souza SandesI; Guacira Corrêa de MatosII; RosanyBochnerIII; Sônia Maria Cezar GóesIV; Elisangela da Costa Lima-DellamoraV
IPharmacist, Specialist in Hospital Pharmacy, Master Student in Applied Science for Health products, Pharmacy School, Federal University Fluminense. Niterói, Brazil. E-mail: valcienyfar@hotmail.com.
IIPharmacist, PhD in Public Health, Associate Professor, Pharmacy School, Federal University of Rio de Janeiro. Brazil. E-mail: gcmatos@globo.com.
IIIStatistics, PhD in Public Health, Public Health Technologist at the Oswaldo Cruz Foundation, Rio de Janeiro, Brazil. E-mail: rosany.bochner@icict.fiocruz.br.
IVNurse, Master in Public Health, Risk Manager of the University Hospital Clementino Fraga Filho, Federal University of Rio de Janeiro. Brazil. E-mail: sonia.cezar.goes@gmail.com.
VPharmacist, PhD in Public Health, Associate Professor, Pharmacy School, Federal University of Rio de Janeiro. Brazil. E-mail: lima.dellamora@gmail.com.
DOI: http://dx.doi.org/10.12957/reuerj.2014.6075
ABSTRACT: Drug interactions (DI) exacerbate the risk of serious adverse events related to the use of benzodiazepines. This study focuses on aspects related to the use of flumazenil in hospitalized patients. Through the observational method, prescriptions and medical records of 31 patients admitted from June 2008 to June2010 were analyzed concerning the indication for flumazenil, factors that may have contributed to poisoning by benzodiazepines and frequency of DI. The frequency of indication for reversal of excessive sedation was approximately 1.3 prescriptions per 1,000 patients. DI were identified in 84% of these prescriptions. In seven cases, there was no prior prescription of benzodiazepines. The method allowed the identification of events requiring the management of excessive sedation associated with the occurrence of potential DI among benzodiazepines and other drugs for a high percentage of patients. It was observed that advanced age; clinical picture with many comorbidities and medication administration with well defined interactions were associated with hipersedation.
Keywords: Flumazenil; drug interactions; adverse events; benzodiazepines.
INTRODUCTION
The risk of using drugs in hospitalized patients and implications related to errors in the care process has gained wide prominence in the literature 1,2. However, despite the severity of the problem, the development and adoption of safe practices by the healthcare team during training and professional performance are hampered by lack of standardization of concepts in addition to underreporting on the occurrence of adverse events and their causal factors3.
The adverse drug events (ADEs) cause health risks to patients causing permanent damage, prolonging hospitalization and increasing health care costs. Methods of monitoring and prevention of these events have been evaluated and used as quality indicators in health systems4,5.
It is known that the simultaneous use of multiple medications is common in clinical practice and is intrinsically related to the risk of drug interactions and adverse reactions6. Among the drugs commonly involved in adverse events, we can highlight the psychotropic7. About formulations with this action, benzodiazepines are used as hypnotics of choice due to its efficacy and safety, being among the most consumed drugs in the world8.
In Brazil, over-consumption of benzodiazepines can be inferred from the fact that this therapeutic class be responsible for the largest number of poisonings. According to the Toxicology Information Center of Rio Grande do Sul, in 2009, 9,271 cases of drug intoxication were recorded, of which 220 occurred unintentionally, by benzodiazepines9.
It is difficult to detect and document adverse events to benzodiazepines. However, the flumazenil utilization study may be useful to indicate the occurrence and estimate the frequency of over-sedation for this therapeutic class10,11. Because this drugs act on the central nervous system, knowledge about the use of flumazenil is particularly important, both with regard to the analysis of the usage profile in an environment of training of health professionals and to identify adverse events and errors medication involved, and in adopting measures to ensure patient’s safety. It is estimated that in Brazil, 4.8 million new cases of accidental or intentional poisoning occur each year, of which 0.1 to 0.4% result in death12. Although many studies address the intentional intoxication with psychotropic drugs, research on the frequency of use of flumazenil in hospitalized patients is scarce in the country.
The aim of this study is to know aspects and elements related to the use of flumazenil in hospitalized patients in a general university hospital, and discuss factors, high-risk groups and clinical situations that have led to excessive sedation and subsequent use of that antidote.
LITERATURE REVIEW
Poisoning by benzodiazepines can cause depression of the central nervous system with varying degree of decrease in level of consciousness, drowsiness, lethargy, excessive sedation, confusion, ataxia, amnesia, respiratory depression, hypotension, hypothermia, bradycardia, and coma, as well as being associated with the risk of falls in the elderly.
Exacerbation of the therapeutic effects of benzodiazepines in co administration with other drugs is common, due to the potential risk of drug interactions that influence the pharmacokinetics of the first and increase their toxicity8.
Drug Flumazenil (8-Fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo [1.5a] [1,4] benzodiazepine - 3-ethyl carboxylate) blocks, by competitive inhibition, the central effects of substances that act at the level of benzodiazepine receptors13. It is classified as an antidote for Anatomical TherapeuticChemical (ATC) under the V03AB25 code. It has no defined daily dose (DDD) and its use is indicated mainly in the reversal of the sedative action after anesthesia as well as in the treatment of acute overdose of benzodiazepines14,15. Other uses, yet insufficiently established, have been described in the literature for improving mental status of patients with severe liver disease (hepatic encephalopathy)16,17 alcohol intoxication18,19 and poisoning by plants of the genus Cannabis20.
METHODOLOGY
An observational study of exploratory, descriptive and retrospective approachwas held. Data from hospitalized patients were analyzed from June 2008 to June 2010, of a federal public hospital, general and large type located in the State of Rio de Janeiro and registered to care for highly complex referenced demand without emergency care21.
All patients admitted to medical beds aged over 18 years, for whom the prescription of flumazenil was required in the period of investigation were included in the study. It was obtained the ratio of patients in the specified condition, from consulting the computerized system for prescribing and electronic medical records of the institution. We excluded patients on postoperative observation due to the routine use of this drug for reversal of sedation of anesthesia15.
Among the 19,355 hospitalized during the observation period used the antidote flumazenil and were selected for the study. Data were collected on computerized medical records, relating to the therapeutic profile, complemented by clinical history, comorbidities diagnosed, tests and procedures performed, age and sex of patients.
The drugs were classified until the fifth level, according to the ATC method outlined by the World Health Organization14. The information, organized in a spreadsheet, was analyzed by quantitative-qualitative approach. We sought to investigate: the indication of flumazenil; the factors that may have contributed to the state of intoxication by benzodiazepines and the frequency of potential drug interactions between benzodiazepines and other prescribed medicines during hospitalization of patients studied.
For qualitative analysis of the use of flumazenil, it was elaborated a theoretical and conceptual framework based on an integrative literature review regarding the indication of the drug22. We used the electronic databases MEDLINE, LILACS, SciELO from the Virtual Health Library (VHL) in the search for studies indexed in Portuguese, English and Spanish from 1990 to 2010. Micromedex® basis was used as reference for identification and classification of severity of interactions23.
The prevalence was obtained as a measure of frequency, calculated from the observed number of cases over the total number of patients admitted in the period.
The study was approved by the Ethics Research Committee (number 742/10).
RESULTS AND DISCUSSION
Factors associated with the prescription and use of flumazenil
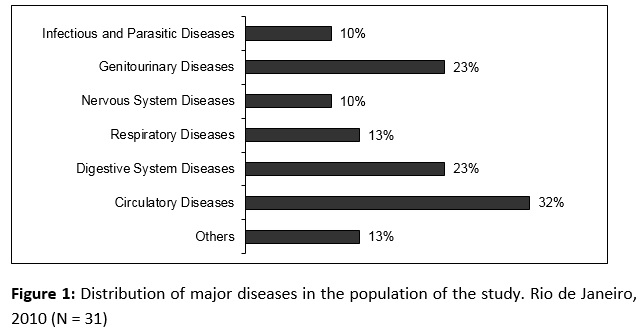
The hospitalization time of 31 selected patients in the study ranged from 8 to 300 days, with a mean of 57 and median 37.5 days. The majority were male (53%) and had more than 60 years old (56.2%). The age range was between 23 and 82 years old, mean 59 ± 19 years and a median of 64 years old. Major diseases and comorbidities that were reported in the medical record are shown in Figure 1.

For most patients, the prescribed dose of flumazenil was 0.5 milligrams in 24 hours after use of benzodiazepine. However, there were situations in which this period extended for up to four days.
Flumazenil was indicated for the treatment of excessive sedation in 24 patients, representing an approximate frequency of 1.3 prescriptions per 1000 patients. In other cases (seven), its use occurred with no record of prior benzodiazepine prescriptions. Among these, four records met the test of flumazenil registered as indication of use, which rested on suspicion of poisoning by benzodiazepines prior to admission. In the case of one patient, there was mention in the records of exacerbating the effect of morphine, suggesting confusion between the indication of antidotes naloxone and flumazenil to reverse the action of opioids and benzodiazepines, respectively. In two other instances, no reason for use was identified. It was not possible, however, to rule out the prescription of benzodiazepines for procedures not recorded in the medical records.
For 84% of patients for whom the prescription of flumazenil was required after benzodiazepine use, we observed the occurrence of potential drug interactions (PDI) due to associations between these sedatives and other medicines used during the hospitalization period. The analysis of the history recorded in the medical records of these patients allowed the identification of competing factors cited in the literature, such as age; health and impaired renal and hepatic function, which may have influenced the susceptibility to drug interactions. Such factors as well as drugs involved in each case, are shown in Table 1.

Frequency of potential drug interactions
Prescribed benzodiazepines belonged to the list of essential medicines in the investigated hospital and had long (diazepam, clonazepam) and short activity (midazolam). These were administered in therapeutic doses for procedures such as intubation, electrical cardioversion and puncture, or even to maintain patient sedation.
The prescription of benzodiazepines because of seizures occurred in four cases, and two of these patients were epileptic. In situations of anesthetic induction, it was observed the association of two benzodiazepines.
Mild to severe drug interactions were found in prescriptions for 20 patients, some with up to three concurrent interactions. The severity, frequency and description of the 36 potential drug interactions effects found were arranged in Table 2.
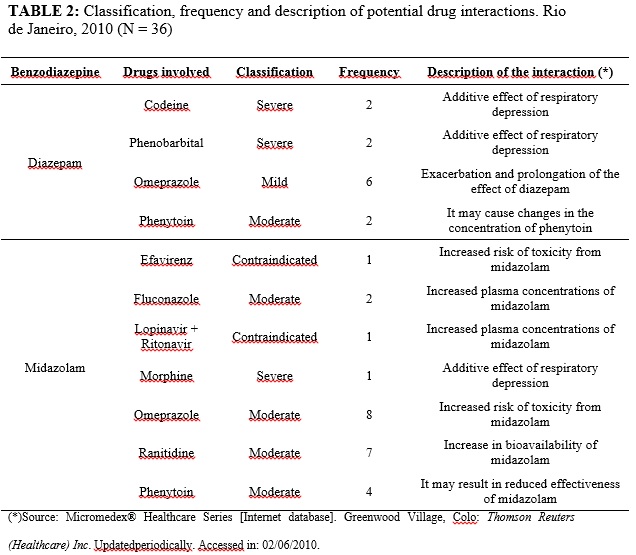
Only in twelve cases, the events arising from the use of benzodiazepines were identified and described in the medical record as excessive sedation with or without reducing the level of consciousness (9), hypotension (1), disorientation (1) and respiratory depression (1).
Flumazenil is not a medicine used routinely in most hospitals. It is indicated for cases of suspected overdose of benzodiazepines and reversal of the sedative effects. The literature describes it as a specific tracer for the detection of adverse drug events (trigger tool)24, which highlights the importance of investigating its use.
A study analyzed the complications caused by all kinds of drugs - adverse effects, errors in prescribing or administration, poisoning and self-harm - in inpatients by the Public Health System in the State of Rio de Janeiro, observing prevalence of 1.8 injuries per 1,000 admissions25, very close to that found in this study, which was 1.3 per 1,000. However, data from this study exclude cases relating to intentional poisonings caused by benzodiazepines, whose frequency is quite significant9, focusing specifically on the events of the therapeutic use of medications during hospitalization at a university hospital that does not have emergency service open to the general population.
The prescription of flumazenil should be cautious and held as strategic behavior when, it is known that overdose is due to benzodiazepines.8. Its empirical administration is questioned in comatose patients due to adverse events that include: cardiac arrhythmia, pain at the injection site, dizziness, nausea, abnormal or blurred vision, restlessness, irritability and resedation23.
Investigation of clinical and drug history of patients allowed the observation of other disagreements in its use. In one case, flumazenil was prescribed for poisoning by morphine, as reported in the medical record, which leads to the assumption that this drug was confused with naloxone, recommended drug for opiate intoxication. Such an event is characterized as a medication error prescribing error type, due to the possibility to cause or induce the inappropriate use of medications and to be preventable26,27.
The administration of flumazenil is contraindicated in patients with increased intracranial pressure and with a history of epilepsy 8. In this study, four patients (12.5%) had used benzodiazepines for the treatment of seizures, two of them related to the diagnosis of severe epilepsy according to clinical report.
An analysis of 43 cases of seizures after use of flumazenil, made in another study, showed that the cause was related to the association with tricyclic antidepressants or unmasking anticonvulsant effect of benzodiazepines28. Clinical trials have indicated that the risk of death is high in the administration of flumazenil in patients taking tricyclic antidepressants in high doses or have serious illnesses8,23.
As for potential drug interactions in exam, most were moderate to high severity. These interactions were associated with complex clinical pictures. It is noteworthy the fact that the biotransformation of long term benzodiazepines and most of short action is subject to individual variations in pharmacokinetic parameters, attributed to factors such as age, liver damage, and concomitant use of enzyme inducers and inhibitors8,10. Benzodiazepines should be avoided in elderly patients by the particular sensitivity of this age group due to reduced hepatic blood flow and reduced enzyme activity29,30. The combination of physiological decline with increasing comorbidities may lead to cognitive impairment, delirium, falls and fractures31. The results of this study reflect this risk, since it was observed patients whose diseases - heart, kidney and hepatic insufficiency - influenced the pharmacokinetics of benzodiazepines, in addition to the hemodynamic instability related to other disorders and the severity of the clinical condition.
The occurrence of severe fractures due to fall in elderly Brazilians who use benzodiazepines is well known in the literature32. Hipersedation exacerbates this risk. Preventing falls is one of the actions envisaged by the recent National Program of Patient Safety (NPPS)33. The protocol for the prevention of falls that integrates NPPS reaffirms the need for attention from nursing staff to the classes of drugs used by the elderly patient33. The interaction with the clinical pharmacist is recommended and contributes to cooperation and exchange of knowledge between the multidisciplinary team regarding the rationalization process of therapy33,34.
Researchers reported that the risk of injury in the population doubles when there is association between benzodiazepines and other drugs35. However, the fact that benzodiazepines are recognized as well-tolerated drugs 8and the existence of a drug to reverse its effects may contribute to minimalizing other risks in its use in the hospital routine.
For this reason, the combination of depressants of the central nervous system in the elderly or in patients with renal, cardiac or hepatic failure shall be monitored, and the use of less than the usual dose is indicated. The effects on the cardiovascular system are usually caused by decreased peripheral vascular resistance, myocardial depression and reduced cardiac output36.
The use of flumazenil has been enrolled, in a higher degree, with the prior administration of midazolam, a sedative widely used in the hospital environment. The fact that this benzodiazepine is involved in most situations that required reversal of sedation is consistent with its classification as a potentially dangerous drug37. Comparing it to other drugs in the same class, midazolam has important intra-individual variability in pharmacokinetics38. Considering the occurrence of potential drug interactions in 84% of cases, it is noteworthy that many clinical situations often require the use of medications known to be conflicting, which complicates the prevention of possible adverse events10. It is suspected that flumazenil is the main alternative to prevent or minimize the damage of these events, at the expense of other strategies such as administration of smaller doses and close clinical monitoring. A study in a university hospital in São Paulo, on the use of midazolam, reported that in 88% of cases which the prescription of flumazenil was required, there were clinically significant drug interactions between benzodiazepines and opiate agonists, furosemide, pancuronium and calcium gluconate10.
In the present work, the interactions with medications used to treat peptic ulcers and gastroesophageal reflux and antiretrovirals were emphasized, because of the observed frequency and contraindications of the association, respectively. It is possible that routine and sometimes inappropriate prescribing of omeprazole and ranitidine for inpatients is the reason for the observed frequency39.
The need of precaution due to the risk of poisoning, reaffirmed in a recent paper on the pharmacodynamics of drugs used for deep sedation30, also emphasize the importance of multi-professional follow-up with active participation2. The monitoring and careful analysis of failures during drug therapy by institutions should contribute to its reduction40. Some efforts to reduce inappropriate use of benzodiazepines have been already reported41 and include educational approaches, alerts, audit and feedback. These authors have emphasized the multidisciplinary interaction among prescribers, pharmacists and nurses of critical importance in improving patient safety.
CONCLUSION
This study has the limitation of not comprising generalizations, since it was conducted exclusively with the population of a state hospital in Rio de Janeiro. The analysis, however, allowed the identification and the need for management of excessive sedation - severe adverse reaction and with important clinical and economic complications - related to the occurrence of potential drug interactions between benzodiazepines and other drugs for a high percentage of patients.
Results showed that the presence of factors such as advanced age; clinical picture with many comorbidities and medication administration with well-defined interactions as well as their characteristics, were associated to the use of flumazenil antidote to reverse sedation, in most cases analyzed.
In this sense, the fact that this reaction is preventable suggests that the teaching hospital studied needs further awareness of the multidisciplinary team regarding strategies for patient safety and risks of drug therapy. The observation of disagreement in the use of flumazenil reinforces the need to increase the production of knowledge on the subject.
The methodological contribution of studies of medication use (a drug indicated as an antidote in the present investigation) was configured as a valuable tool for the observation and identification of events associated with the use of benzodiazepines during the process of health care. The severity and the effects of these interactions were relevant, which encourages new studies on the use of drugs acting on the central nervous system as well as the rigorous therapeutic monitoring.
REFERENCES
1.Rosa MB, Perini E, Anacleto TA, Neiva HM, Bogutchi T. Erros na prescrição hospitalar de medicamentos potencialmente perigosos. Rev Saúde Pública. 2009; 43: 490-8.
2.Lopes DMA, Néri EDR, Madeira LS, Souza Neto PJ, Lélis ARA, Souza TR, et al. Análise da rotulagem de medicamentos semelhantes: potenciais erros de medicação. RevAssocMed Bras. 2012; 58(1): 95-103.
3.Silva AEBC. Segurança do paciente: desafios para a prática e a investigação em enfermagem. Rev Eletr Enf. 2010; 12(3): 422.
4.Naessens JM, Campbell CR, Huddleston JM, Berg BP, Lefante JJ, Williams AR, Culbertson RA. A comparison of hospital adverse events identified by three widely use detection methods. Int J Qual Health Care. 2009; 21: 301-7.
5.Souza LP, Bezerra ALQ, Silva AEC, Carneiro FS, Paranaguá TTB, Lemos LF. Eventos adversos: instrumento de avaliação do desempenho em centro cirúrgico de um hospital universitário. Rev enferm UERJ. 2011; 19: 127-33.
6.Secoli SR. Polifarmácia: interações e reações adversas no uso de medicamentos por idosos. Rev. Bras de Enfermagem. 2010; 63: 136-40.
7.Forster AJ, Halil RB, Tierney MG. Pharmacist surveillance of adverse drug events. Am. J. Health-System Pharm. 2004; 61:1466-72.
8.Oga S, Camargo MMA, Batistuzzo JAO. Fundamentos da toxicologia. 3ª ed. São Paulo: Atheneu, 2008.
9.Centro de Informação Toxicológica do Rio Grande do Sul. Secretaria Estadual de Saúde. Toxicovigilância-Toxicologia Clínica: dados e indicadores selecionados – 2009/2010. Porto Alegre (RS): CIT-RS; 2011.
10.Kawano FD, Ueta J, Sankarankutty AK, Pereira LRL, Freitas O. Midazolam-related drug interactions: detection of risk situations to the patient safety in a Brazilian teaching hospital. J Patient Saf. 2009; 5(2): 69-74.
11.Naessens JM, O’byrne TJ, Johnson MG, Vansuch MB, Mcglone CM, Huddleston JM. Measuring hospital adverse events: assessing inter-rater reliability and trigger performance of the Global Trigger Tool. Int J Qual Health Care. 2010; 22: 266-74.
12.Zambolim CM, Oliveira TP, Hoffmann NA, Vilela CEB, Neves D, Anjos FR, et al. Perfil das intoxicações exógenas em um hospital universitário. Revista Médica de Minas Gerais. 2008; 18(1): 5-10.
13.Karavokiros KA, Tsipis GB. Flumazenil: a benzodiazepine antagonist. DICP.1990; 24:976-81.
14.WHOCC – World Health Organization Collaborating Centre for Drug Statistics Methodology. [site de Internet] [cited in 2014 apr 12]. Disposable in: www.whocc.no/
15.Agencia Nacional de Vigilãncia Sanitária. Flumazenil. [citado em 15 fev 2014]. Disponível em: www.anvisa.gov.br /base/visadoc/BM/BM[26097-1-0].
16.Als-Nielsen B, Gluud LL, Gluud C. Benzodiazepine receptor antagonists for hepatic encephalopathy. Cochrane database Syst Rev. 2001; (4): CD002798.
17.Kuromashiro R. Treatment of minimal hepatic encephalopathy. Hepatol Res. 2008; (38): S128-31.
18.Anton RF, Myrick H, Baros AM, Latham PK, Randall PK, Wright TM et al. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J ClinPsycopharmacol.2009; 29: 334-42.
19.Soyka M, Rösner S. Emerging drugs to treat alcoholism. Expert Opin Emerg Drugs. 2010;15(4):695-711.
20.Lassaletta A, Martino R, Gónzalez-Santiago P, Torrijos C, Cebrero M, García-Frías E. Reversal of na antihistamine-induced coma with flumazenil. Pediatr Emerg Care. 2004; 20: 319-20.
21.Cadastro Nacional de Estabelecimentos de Saúde (Br). [site de Internet] [citado em25 jun 2011]. Disponível em:www.cnes.datasus.gov.br.
22.Castro CGSO. Estudos de utilização de medicamentos: noções básicas. Rio de Janeiro: Fiocruz; 2000.
23.Micromedex® Healthcare Series [site de Internet]. Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc. Updated periodically. [citado em 02jun2014]. Disponível em: http://www.micromedex.com
24.Rozich JD, Haraden CR, Resar RK. Adverse drug event trigger tool: a pratical methodology for measuring medication related harm. QualSaf Health Care. 2003; 12(3): 194-200.
25.Rozenfeld S. Agravos provocados por medicamentos em hospitais do Estado do Rio de Janeiro, Brasil. Rev Saude Publica. 2007; 41: 108-15.
26.Silva AEBC, Cassiani SHBC. Erros de medicação em uym hospital universitário: tipo, causas, sugestões e providências. Rev Bras Enferm. 2004; 57:671-4.
27.National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). Whatis a medication error; 1998.[site de Internet]. [cited in 2011nov 12] Disponível em: http://www.nccmerp.org/medErrorTaxonomy.html.
28.Spivey WH. Flumazenil and seizures: analysis of 43 cases. Clin Ther. 1992; 14: 292-305.
29.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the beers criteria for potentially inappropriate medication use in older adults results of a US consensus panel of experts. Arch Intern Med. 2003;163:2716-25.
30.Becker DE. Pharmacodynamic considerations for moderate and deep sedation.AnesthProg.2012; 59:28-42.
31.Cresswell KM, Fernando B, McKinstry B, Sheikh A. Adverse drug events in the elderly. British Medical Bulletin.2007; 83: 259-74.
32.Coutinho ESF, Silva SD. Uso de me medicamentos como fator de risco para fratura grave decorrente de queda em idosos. Cad Saúde Pública. 2002; 18:1359-66.
33.Ministério da Saúde (Br). Portaria nº 529, de 1º de abril de 2013. Institui o Programa Nacional de Segurança do Paciente (PNSP). Brasília (DF): Gabinete Ministerial; 2013.
34.Matos E, Pires DEP, Gelbcke FL. Implicações da interdisciplinaridade na organização do trabalho da enfermagem: estudo em equipe de cuidados paliativos. Rev Eletr Enf. [Internet]. 2012; 14:230-9.
35.French DD, Chirikos TN, Spehar A, Campbell R, Means H, Bulat T. Effect of concomitant use of benzodiazepines and other drugs on the risk of injury in a veterans population. Drug Safety. 2005; 28:1141-50.
36.Cury AF, Vieira MLC, Fischer CH, Rodrigues ACT, Cordovil A, Monaco C et al. Segurança da ecocardiografia transesofágica em adultos. Estudo em um hospital multidisciplinar. Arq Bras Cardiol 2009;93:478-83.
37.ISMP. Institute for safe medication practices.[site de Internet]. [cited in 2014 jun 10]. Disposible in:www.ismp.org.
38.Swart EL, Zuideveld KP, Jongh J, Danhof M, Thijs TG, Schijndel RMJS. Comparative population pharmacokinetics of lorazepam and midazolam during long-term continuous infusion in critically ill patients.Br J ClinPharmacol.2004; 57:2 135-45.
39.Munari L, HartD, Morrone FB.Uso de omeprazol em hospital universitário de Porto Alegre-RS (Brasil). Seguimiento Farmacoterapéutico. 2004; 2: 235-43.
40.Rosa EB, Perini E. Erros de medicação: Quem foi? Rev Assoc Med Bras. 2003; 49: 335-41.
41.Smith AJ, Tett SE. Improving the use of benzodiazepines – is it possible? A non-systematic review of interventions tried in the last 20 years. BMC Health Serv Res. 2010; 10: 321.