RESEARCH ARTICLES
Chlorhexidine-impregnated dressing for central venous catheter: pilot clinical trial
Edivane PedroloI; Michelle Caroline SantosII; Gabriella Lemes Rodrigues de OliveiraIII; Priscila MingoranceIV; Mitzy Tannia Reichembach DanskiV; Radamés BoostelVI
INurse. Master degree in Nursing. Federal Institute of Paraná. Curitiba, Paraná, Brazil. E-mail: edivane.pedrolo@ifpr.edu.br.
IINursing student in the Federal University of Paraná. Curitiba, Paraná, Brazil. E-mail: chelli_carol@hotmail.com.
IIINurse. Master degree student in Nursing from the Graduate Program in Nursing from the Federal University of Paraná. Curitiba, Paraná, Brazil. E-mail: gabriella.lemes@yahoo.com.br.
IVMaster degree in Nursing. Federal University of Paraná. Curitiba, Paraná, Brazil. E-mail: primingo@yahoo.com.br.
VNurse. Ph.D. in History. Nursing Department of the Federal University of Paraná. Curitiba, Paraná, Brazil. E-mail: profa.mitzy@ufpr.br.
VISpecialist Nurse in Urgency and Emergency. Federal Institute of Paraná. Curitiba, Paraná, Brazil. E-mail: radames.boostel@ifpr.edu.br.
VIIArticle extracted from the dissertation entitled chlorhexidine dressing for central venous catheter: randomized clinical trial, presented at the Graduate Program in Nursing from the Federal University of Paraná.
DOI: http://dx.doi.org/10.12957/reuerj.2014.5547
ABSTRACT: This randomized pilot clinical trial aimed to evaluate the effectiveness of chlorhexidine-impregnated dressings covering central venous catheters. Data was collected between October and November 2011 at the adult intensive and semiintensive care units of a university hospital in Curitiba, Paraná, Brazil. The eight patients included in the trial were randomized into two groups: transparent polyurethane film dressing (control) and chlorhexidine-impregnated dressing. There was one case of blood stream infection associated with the catheter and one of catheter colonization, both in the control group. The transparent polyurethane film needs to be changed earlier than specified, due to poor fixation and the accumulation of exudate under the film, which can expose patients to greater risk of colonization and infection of the blood stream associated with the catheter.
Keywords: Clinical trial; chlorhexidine; central venous catheter; technology.
INTRODUCTION
The hospitalization of patients in Intensive Care Units (ICU) and Semi Intensive (ITSI) requires the use of advanced technologies for therapy and care, including intravenous devices with emphasis on central venous catheter (CVC). However, the use of central catheters is related to high rates of morbidity and mortality, which requires the adoption of measures for the prevention of complications, especially the infectious complications.
In this context, new technologies have emerged in recent years in order to reduce the rates of infectious complications related to catheters. Among them, there are the specific dressing development to coverage of the ostium of the catheter as the impregnated dressing with chlorhexidine, an antiseptic solution widely employed in the hospital environment. However, the adoption of this curative as material of choice for central catheters coverage requires scientific evidence, which can be produced with the completion of a randomized clinical trial. With all this, the present research aimed to perform a pilot test of a clinical research, elaborated to evaluate the effectiveness of the impregnated dressing with chlorhexidine to cover central venous catheter.
LITERATURE REVIEW
The CVCs are devices widely used due to the benefits they present. However, they are related to a series of complications, both infectious and non-infectious. The infections can occur during insertion and/or maintenance of the catheter1.
Among the infectious complications, there is the Primary Infection of the Bloodstream (PIBS), which is commonly related to the use of the CVC, prolonging the time of hospitalization of patients and raising spending on their treatment. The PIBS is a serious systemic infection without identifiable primary focus. It can be related to performance of any invasive procedure, however it is considered that when it occurs in patients with use of CVC for more than 48 hours, it is related to the presence of this device. The diagnosis of the PIBS can be established based on inflammatory clinical signs, (local and systemic) or from laboratory data, where the microbiological infection is confirmed. It is highlighted the importance of this complication, since about 69% of patients hospitalized in ICU die due to the occurrence of PIBS2.3.4.
In order to prevent this complication, some care are needed during and after the insertion of the device. Due to the solution of continuity in the ostium of the catheter, it must be protected with occlusive sterile dressing4. Currently, the following materials are available on the market: gauze bandages and adhesive tape, transparent film of polyurethane (TFP) and impregnated with chlorhexidine1.5.
When compared with the gauze and tape dressing, TFP is better because of its bigger length of stay, reducing the cost of materials and the possibility of observing the ostium of the catheter without the need of removing this dressing5. Among the impregnated dressings, the antimicrobial dressing of chlorhexidine (CHG), composed of transparent film associated to chlorhexidine 2%, concentrated in a gel plate or in a sponge, depending on the manufacturer. This dressing also has the advantage of observation of the ostium of the catheter without having to remove it6.
It is highlighted that the gauze and tape and transparent polyurethane have been widely studied in recent years, not having evidence indicating the use of one over the other. The studies about impregnated dressing with chlorhexidine are incipient4.7.
METHODOLOGY
The present research was approved by the Research Ethics Committee of a Federal University in the city of Curitiba-PR, CEP/SD record under 1145.070.11.06 and CAAE 0067.0.091.208-11. It is the pilot testing of a randomized clinical trial.
Data collection occurred in the ICU and adult CTSI of a University Hospital in the city of Curitiba-PR, in the months of October and November 2011. The patient inclusion in the research was according to the following inclusion criteria: signature of informed consent (TFCC); being eighteen years old; hospitalization in the ICU or CTSI adult; using the first CVC of short stay less than 24 hours; absence of known sensitivity to dressings materials. Exclusion criteria were: submission to the blade Trichotomy in puncture catheter insertion; presence of infectious blood before the catheter puncture.
Patients who had the inclusion criteria were randomized through randomization technique with six individuals8, to compose the control groups (transparent polyurethane dressing - TFP) and study (chlorhexidine antimicrobial dressing - CHG).
There was daily monitoring and dressing exchange every seven days for both groups, or prematurely in case of impairment of integrity by detachment of borders, the presence of exudate, moisture or external dirt. Exclusively qualified professionals carried out data collection and dressing exchange, after standardization of the procedure to be employed.
Socio-demographic variables, clinical and outcome according to a data collection instrument previously prepared were collected. Outcome variables analyzed were: primary infection of the bloodstream, local reaction to dressing and dressing fixing. For data analysis, descriptive statistics were used. The data were entered and tabulated in spreadsheet in Microsoft Office Excel® 2007.
RESULTS AND DISCUSSION
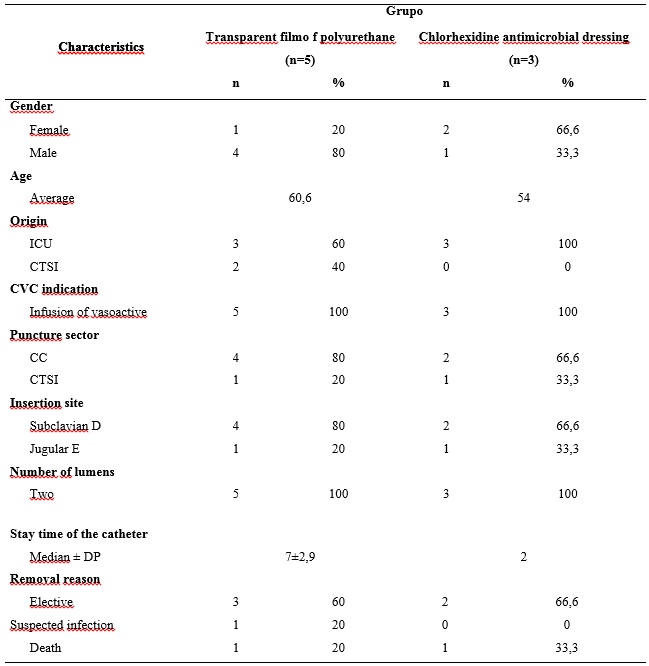
The sample consisted of eight patients, five from the control group (TFP) and three of the study group (CHG). Most of them were hospitalized in the ICU (6). Of the total number of patients included, five were male and Caucasian origin, with an average age of 58.1 years old, minimum of 37 and maximum of 91 years old, according to Table 1.
Regarding clinical variables, the average time of hospitalization of patients was 10.1 ± 7.2 days, with a minimum of two and maximum of 24. Most patients (five) were discharged, while the remaining (three) died.
For collection of the diagnoses, it was considered the primary diagnosis at the time of hospitalization of patients, listed according to the International Classification of Diseases (ICD-10)9. There was a predominance of diseases related to the genitourinary system (37%) and the circulatory system (25%).
Fourteen comorbidities were found among the subjects of the research. Two patients did not present comorbidities, however most of them had two or more pathologies, which justifies the high number of comorbidities found. The main existing comorbidities were hypertension (28.6%) and diabetes mellitus (21.4%) diseases also frequent found under a study that described the profile of patients hospitalized to adult intensive care unit (n=37)10.
Of the total of patients analyzed, four had prescription antimicrobial therapy before to the puncture of the CVC. From them, three patients used only one antimicrobial, while one used two of them. In the literature, there are not reports of the relationship between antimicrobial use and increased risk of infectious complications.
Regarding the characteristics related to the catheter, all were of double lumen, made of polyurethane and inserted to infusion of vasoactive. Researchers claim that each lumen increases of 15 to 20 times/day the catheter manipulation, thereby increasing the risk of infection11. In an epidemiological study conducted with 630 patients, in which catheters mono were included, dual and triple lumen, it was noted the occurrence of 40 cases of PIBS, 85% related to the use of double lumen catheters12.
The general average of CVC permanence was 5.1 days, with median of 5.5, minimum of one and a maximum of 10 days. The authors indicate the permanence of the catheter for more than five days as a risk factor for the development of PIBS13. The present research presented a case of PIBS, which is identified in a subject with use of CVC for seven days.
As regards the sector of catheter puncture, six were punctured in the Surgical Center (SC) and two in CTSI. The insertion sites were the right subclavian vein and left jugular, with six and two respectively. It is observed a preference of the insertion of the catheter in the right subclavian vein and left jugular, as demonstrated in Table 1. However, there is no safety evidence relating the two places with the presence of infection14. The recommendation for a higher level of scientific evidence guides the insertion of catheters for short stay preferably in the subclavian vein, as well as avoiding the femoral vein puncture for this type of device4.
In the removal of the catheter, a patient had his retreat on suspicion of infection (unconfirmed), two were died and five had elective removal of catheter, that is, the device was no longer needed, according to Table 1.
TABLE 1: Characteristics of patients and the catheters in researched groups, Curitiba, 2012

The length of stay of the two types of dressing was similar, with an average of 2.5 days for the transparent film and two days for the dressing of CHG. It is highlighted that only the transparent polyurethane film dressing required advance exchange, of which six were due to poor installation and two by abundant exudate.
For variables related to infection (fever, hypotension, oliguria, cyanosis, and hyperemia exudation in the ostium of the catheter, induration, pain or swelling on palpation) and local reaction (itching, hyperemia, flaking and maceration), patients who presented these signs were at least 70% of the days of monitoring. Only two variables were identified in only one patient: the presence of skin in hyperemia and in the ostium of insertion. This divergent data from randomized clinical trial conducted with 21 patients, evaluating the transparent dressing as cover for CVC, which the hyperemia was the third signal more often observed in 33% of pacients7.
As for the ability of fixing the transparent film, it was considered good in 15 days, bad in seven days and detached edge presence in 11 days, totaling 33 days of monitoring. The transparent polyurethane dressing required advance exchange, in relation to international recommendations4, due to the presence of exudate (two) and poor installation (five). For the dressing of CHG, fixation was considered good in every day monitoring.
The average time of CHG dressing was two days, while the transparent dressing was 2.5 days. The results are similar as another study (n=21), which presents the average stay of 1.9 days for transparent dressing7. It is highlighted that the time of permanence of the chlorhexidine dressing is up to seven days. However, in this research it was lower due to the length of stay of the catheter in the group of patients who made use of this technology, which was two days on average.
There was a case of bloodstream infection associated with the catheter and one case of catheter colonization, with isolation of the microorganism coagulase-negative Staphylococcus. This data are similar to studies that bring Gram-positive bacteria as the main etiological agents of PIBS, being coagulase-negative Staphylococcus as the most frequent4.15-16.
Of the total of eight patients, three of them used the chlorhexidine antimicrobial dressing. Meta-analysis showed a reduction of 60% of infections associated with CVC when using this dressing17. In addition, tests of skin cultures of patients using dressings of CHG demonstrate a significant reduction of the presence of microorganisms with pathogenic potential compared to conventional transparent dressing.
In the national literature there are still no studies compared to chlorhexidine antimicrobial dressing. In the international literature, randomized trial compared this dressing with transparent film of polyurethane, noting a reduction in the cases of PIBS with the use of the curative of CHG18.
The insertion of technologies and innovations in the field of nursing is a necessity and a reality. However, despite technologies bring a number of advantages for the nursing work, including greater safety in care19, there is a need to evaluate its effectiveness. In this way, the present research contributes to incorporation of a technological innovation for the care of the central venous catheter, in order to reduce the rate of infectious complications related to this device, which saddled both services as the health condition of the patients.
CONCLUSION
The type of dressing used to cover the catheter interfered in the final results. The outcomes of primary infection of the bloodstream and catheter colonization occurred in patients who used the transparent dressing. As for fixing capacity, the result with the dressing of CHG is more satisfactory, since the transparent dressing requires exchange early than expected, due to poor installation and to the accumulation of exudate under the film, exposing patients to an increased risk of colonization and infection of the bloodstream attached to the catheter.
It should be noted as this research limitations, the small sample, being possible the statistical proof of data. However, it complied with the purposes of a pilot test and the data concerning the colonization and PIBS were very important in reformulating the clinical research protocol.
REFERENCES
1.Harada MJCS, Pedreira MLG, organizadoras.Terapia intravenosa e infusões. São Caetano do Sul (SP): Yendis; 2011.
2.Ministério da Saúde (Br). Agência Nacional de Vigilância Sanitária. Unidade de Investigação e Prevenção das Infecções e dos Efeitos Adversos – UIPEA. Corrente Sanguínea: critérios nacionais de Infecção relacionadas à assistência à saúde. Brasília (DF): Ministério da Saúde; 2009.
3.Ministério da Saúde (Br). Agência Nacional de Vigilância Sanitária. Unidade de Investigação e Prevenção das Infecções e dos Efeitos Adversos – UIPEA. Orientações para prevenção de infecção primária de corrente sanguínea. Brasília (DF): Ministério da Saúde; 2010.
4.O’Grady NP, Alexander M, Burns LA, Dellinger P, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers of disease control and prevention (CDC) 2011 [citado em set 2014]. 52(9):1-83. Disponível em: http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf
5.Silveira R, Braga FTMM, Garbin LM, Galvão CM. O uso do filme transparente de poliuretano no cateter venoso central de longa permanência. Rev Latino-Am Enferm. 2010 [citado em set 2014]. 18(6). Disponível em: http://www.scielo.br/pdf/rlae/v18n6/pt_23.pdf
6.Oliveira GLR. Perfil epidemiológico dos pacientes em uso do cateter venoso central em um centro de terapia intensiva [monografia]. Curitiba (PR): Universidade Federal do Paraná; 2012.
7.Pedrolo E, Danski MTR, Mingorance P, De Lazzari LSM, Johann DA. Clinical controlled trial on central venous catheter dressings. Acta Paul Enferm. 2011 [citado em set 2014]. 24(2):278-83. Disponível em: http://www.scielo.br/pdf/ape/v24n2/19.pdf
8.Hulley SB, Cumming SR, Browner WS, Grady DG, Newman TB. Delineando a pesquisa clínica: uma abordagem epidemiológica. 3ª ed. Porto Alegre (RS): Artmed; 2008.
9.Organização Mundial da Saúde. Classificação Estatística Internacional de Doenças e Problemas Relacionados à Saúde (CID-10). 10ª rev. São Paulo: Universidade de São Paulo; 2008 [citado em 11 jun 2012]. Disponível em: <http://www.datasus.gov.br/cid10/v2008/webhelp/cid10.htm>.
10.Marques Netto S, Echer IC, Kuplich NM, Kuchembecker R, Kessler F. Infecção de cateter vascular central em pacientes adultos de um centro de terapia intensiva. Rev Gaúcha Enferm. 2009 [citado em set 2014]. 30(3): 429-36. Disponível em: http://seer.ufrgs.br/RevistaGauchadeEnfermagem/article/view/8957/6964.
11.Associação Paulista de Estudos e Controle de Infecção Hospitalar (APECIH). Infecção relacionada ao uso de cateteres vasculares. São Paulo: APECIH; 2005.
12.Mesiano ERAB, Merchán-hamann M. Infecções da corrente sanguínea em pacientes em uso de cateter venoso central em unidades de terapia intensiva Rev Latino-Am Enferm. 2007 [citado em set 2014]. 15(3). Disponível em: http://www.scielo.br/pdf/rlae/v15n3/pt_v15n3a14.pdf.
13.Porto JP, Dantas RCC, Freitas C, Matoso DC, Almeida AB, Gontijo Filho PP, et al. Bloodstream infection associated/related to the central venous catheter in mixed ICU of adults from a Brazilian university hospital: etiology, pathogenesis and risk factors. Rev Panam Infectol. 2010 [citado em set 2014]. 12(2):24-9.Disponível em: http://www.revista-api.com/2010/pdf/02/API_02_10_D.pdf.
14.Siqueira GLG, Hueb W, Contreira R, Nogueron MA, Cancio DM, Caffaro RA. Infecção de corrente sanguínea relacionada a cateter venoso central (ICSRC) em enfermarias: estudo prospectivo comparativo entre veia subclávia e veia jugular interna. Rev Vasc Bras. 2011 [citado em set 2014]. 10(3): 211-6. Disponível em: http://www.scielo.br/pdf/jvb/v10n3/05.pdf.
15.Ross C, Quesada RMB, Girardello R, Rogeri LMS, Calixto LA, Pelayo JS. Análise microbiológica de pontas de catéteres venosos centrais provenientes de pacientes internados no Hospital Universitário da Universidade Estadual de Londrina. Semina. 2006 [citado em set 2014]. 27(2):117-23. Disponível em: http://www.uel.br/revistas/uel/index.php/seminabio/article/view/3506/2843.
16.Bernardi ACA, Pizzolitto EL, Pizzolitto AC. Detecção da produção de slime por estafilococos coagulase-negativa isolados de cateter venoso central. Rev Ciênc Farm Básica Apl. 2007 [citado em set 2014]. 28(1):57-66. Disponível em: http://200.145.71.150/seer/index.php/Cien_Farm/article/viewFile/346/331.
17.Ho KM, Litton E. Use of chlorhexidine-impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta-analysis. J Antimicrob Chemoth. 2006 [citado em set 2014]. 58(2):281-7. Disponível em: http://jac.oxfordjournals.org/content/58/2/281.full.pdf.
18.Timsit JF, Schwebel C, Bouadma L, Geffroy A,Garrouste-Orgeas M, Pease S, et al. Chlorhexidine-impregnated sponges andlessfrequentdressingchangesforprevention ofcatheter-relatedinfections incritically ill adults: a randomized controlled trial. JAMA. 2009 [citado em set 2014]. 301(12): 1231-41. Disponível em: http://jama.jamanetwork.com/article.aspx?articleid=183597.
19.Salvador PTCO, Oliveira RKM, Costa TD, Santos VEP, Tourinho FSV. Tecnologia e inovação para o cuidado em enfermagem. Rev Enferm UERJ. 2012 [citado em set 2014]. 20:111-7. Disponível em: http://www.facenf.uerj.br/v20n1/v20n1a19.pdf.