RESEARCH ARTICLES
Hickman catheters in hematopoietic stem cell transplantation: surgical implantation, removal and nursing care
Hélen Francine RodriguesI; Lívia Maria GarbinII; Laís Esparrachiari Carvalho CastanhoIII; Belinda Pinto SimõesIV; Ana Carolina de Jesus Vieira CurcioliV; Renata Cristina de Campos Pereira SilveiraVI
I
Nurse. Master's Student in Nursing at the Ribeirão Preto College of Nursing
at the University of São Paulo. Ribeirão Preto, São Paulo, Brazil. E-mail: hfrodrigues.usp@gmail.com
II
Nurse. PhD in Health Sciences and Specialist in Hematology. Nurse, Ribeirão
Preto College of Nursing at the University of São Paulo. Ribeirão Preto,
São Paulo, Brazil. E-mail: liviagarbin@usp.br
III
Nurse. MSc. Professor of Nursing - CENSA Institutes of Higher Education.
Campos dos Goytacazes, Rio de Janeiro, Brazil. E-mail: lais.castanho@gmail.com
IV
Physician. PhD and Professor of Internal Medicine at the Ribeirão Preto
Medical School at the University of Sao Paulo. Ribeirão Preto, São Paulo,
Brazil. E-mail: bpsimoes@fmrp.usp.br
V
MSc. Nurse, Bone Marrow Transplantation Unit at the Clinical Hospital of
the Ribeirão Preto Medical School at the University of São Paulo. Ribeirão
Preto, São Paulo, Brazil. E-mail:
carol_curcioli1@hotmail.com
VI
Nurse. PhD and Professor, Department of General and Specialized Nursing at
the Ribeirão Preto Medical School at the University of São Paulo. Ribeirão
Preto, São Paulo, Brazil. E-mail: recris@eerp.usp.br
DOI: http://dx.doi.org/10.12957/reuerj.2015.4995
ABSTRACT
This cross-sectional study aimed to describe the surgical implantation and removal of Hickman catheters and how this interfaces with the nursing care provided to patients undergoing allogeneic hematopoietic stem cell transplantation. Data were collected from the medical records of 71 patients, totaling 77 (100%) catheters implanted between 2006 and 2010. Surgery was performed in 72 (93.5%) cases by vascular surgery residents, with 63 (81.8%) patients receiving local anesthesia, after antisepsis with alcohol/chlorhexidine. In 42 patients, the conditioning regime began less than 72 hours after catheter implantation. Catheter-related infection led to the removal of 20 (25.9%) devices. Nursing care must focus on patient preparation for catheter implantation, in addition to subsequent interventions such as dressing and handling using aseptic techniques. In conclusion, starting the conditioning regime earlier than recommended may contribute to early catheter removal due to infection of the surgical site. A nursing team trained to identify complications is needed for safe handling and maintenance of the device.
Keywords: Nursing care; hematopoietic stem cell transplantation; bone marrow transplantation; catheterization, central venous.
INTRODUCTION
In recent decades, hematopoietic stem cell transplantation (HSCT) has become an important alternative treatment for various types of malignant and non-malignant hematological, oncological and immunological diseases 1.
Performing HSCT requires the insertion of a long-term central venous catheter (CVC), which enables extended access to the vascular system 2. The Hickman catheter is recommended for patients undergoing allogeneic HSCT because it has the features that this treatment requires, including safe infusion of hematopoietic stem cells3-5.
However, although necessary, the use of this device is associated with a series of complications that may be related to both the surgical implant and its handling and maintenance.
The aim of this study was to describe the surgical implantation and removal of the Hickman catheter, and its interfaces in the nursing care of patients undergoing HSCT.
LITERATURE REVIEW
Hematopoietic stem cell transplantation involves infusion of hematopoietic stem cells (HSC), and can be classified as autologous when the HSC are sourced from the patient himself; syngenic when the donor is an identical twin; and allogeneic when the cells come from another donor6.
Before infusion of HSC, the patient is subjected to the conditioning regimen, the intention of which is to suppress the bone marrow and decrease the risk of rejection of the donor's cells, in the case of allogeneic transplantation7.
The Hickman catheter was developed specifically to meet the venous demands of HSCT, and was initially considered the vascular access option for this therapy8. This catheter is surgically inserted under local or general anesthesia1,3,9. A subcutaneous tunnel that links the place of exit of the CVC on the skin to the point of venipuncture 10 is made, the path of which is a cuff that attaches to the subcutaneous tissue after inflammatory and fibrous reaction of this tissue, determining the definitive setting of the catheter1, 3, 10.
Early post-implantation complications from the catheter include infection, occlusion, embolism and thrombosis11, with catheter-related infection being the most frequent5,12.
Allogeneic HSCT in particular involves a greater risk of infection due to disruption of skin integrity with the CVC implantation, along with delay in wound healing, presence of prolonged neutropenia, use of immunosuppressive drugs and long dwell time of the catheter13.
To control and prevent such complications, judicious care related to insertion, maintenance and handling of the catheter is needed13. Because nurses are the main professionals who handle CVCs, their technical and scientific knowledge and safe handling of this device are indispensable to prevent complications and immediately identify their signs and symptoms 1,13,14.
Patient care based on scientific principles guides the work of modern nursing, and is essential for decision making. Therefore, evidence-based practice is considered an effective strategy for improving health care, using research findings for professional practice15.
METHODOLOGY
This was a retrospective, descriptive study developed in an inpatient unit for allogeneic HSCT in a general public hospital in the state of São Paulo in Brazil. The study population was composed of patients undergoing allogeneic HSCT, who had a Hickman catheter surgically implanted between January 2006 and December 2010. These patients' charts were used for data collection.
Inclusion criteria were: being aged less than 18 years on the day of surgery, having performed the first allogeneic HSCT and undergone implantation of the Hickman catheter under aseptic conditions in the operating room. Initially, 74 patient charts were selected, of which three were excluded because they had incomplete information.
An instrument was developed for data collection, which was submitted to face and content validation, and contained items on clinical characterization (basic pathology, type and start date of conditioning regimen), demographics (gender and age) and on the Hickman catheter (implantation date, duration of surgery, surgeon's specialty, type of anesthesia, antiseptic, vein punctured, operating room complications, date and reason for removal of the catheter).
This research project was approved under case no. 4911/2010 by the Research Ethics Committee of the institution where the study took place.
The collected data were entered into spreadsheets of the Microsoft Office Excel version 2010, with double typing and subsequent validation, and then transferred to the Statistical Package for the Social Sciences (SPSS) software, version 15.0 for Microsoft Windows. All data were analyzed by means of descriptive statistics.
RESULTS AND DISCUSSION
Data were collected from the medical records of 71 patients who underwent allogeneic HSCT, totaling 77 (100%) surgically implanted Hickman catheters. This fact is worrisome, because implantation of the Hickman catheter is considered the first preparatory action for HSCT, when the patient has a good clinical condition12. If implantation of a new catheter is necessary, the procedure will be in more unfavorable clinical conditions, mainly due to the state of immunosuppression of the patient after implantation of the catheter, on the occasion of the start of the conditioning regimen and should therefore be avoided3.
Of the total sample, 41 (57.7%) patients were male. The median age of all subjects was 37 years, with a variation from 18 to 65 years. As for the basic pathology, the most frequent was acute myeloid leukemia (AML), totaling 21 (29.6%) cases. The combination of fludarabine and busulfan was the most frequent conditioning regime, employed in 18 (25.3%) patients, and the combination of busulfan to other conditioning regimens was a characteristic common to 49 (69%) patients.
These results showed a reduced frequency of patients with more advanced ages. In view of the high morbidity and mortality rates related to conditioning regimens traditionally employed, these were considered very aggressive for elderly patients7. However, currently, the use of non-myeloablative or conditionings with reduced intensity such as the combination of fludarabine and busulfan in low doses, has allowed the use of this therapy also in this population16.
As regards the main medical diagnosis, this study is in line with other research17 because it found that AML is the basic pathology that most often receives recommendation for allogeneic HSCT, considering adult patients.
As regards the conditioning regimen, it should be noted that the alkylating agent busulfan is a medication with cytotoxic activity that acts on the precursors of granulocytes in bone marrow18, with both myeloablative and immunosuppressive effects. Moreover, it is an agent associated with systemic dermatologic toxicity, predisposing the patient to dermatitis, erythema, and skin dryness and fragility19, adverse effects characteristic of the treatment.
The 77 (100%) catheter implantations were carried out in the operating room. Whereas infections are the main complication related to use of CVC, one study20 points out that care for prevention and control of this complication must start before its insertion, and the team should use maximum barrier precautions during the procedure. Therefore, since all the procedures were carried out in the operating room, where every barrier precaution is adopted, the results of this study are similar to those advocated in the literature.
The mean duration of surgical implantation was 52.75 minutes (ranging from 10 to 135 minutes), with two procedures performed in a period less than 20 minutes, 46 between 20 and 59 minutes, 24 between 60 to 99 minutes, and 5 procedures in more than 100 minutes.
Greater handling and procedure time can expose the patient to a higher incidence of complications. The duration of surgery recommended by some scholars3 is between 20 to 40 minutes, and can be more than two hours in difficult cases. This study identified an operating time of more than 40 minutes in 52 (67.5%) cases, five of which lasted more than two hours.
Of the 77 implantations, 72 (93.5%) were performed by contracted physicians or resident physicians of vascular surgery, 63 (81.8%) of which were under local anesthesia and preceded by antisepsis with antibacterial chlorhexidine and alcohol. Antibacterial and alcoholic solutions of polyvinyl pyrrolidone iodine (PVPI) were used concurrently in only one procedure. In the other 13 cases only one of the solutions, i.e. antibacterial or alcohol, was used.
Concern that the professional responsible for implantation of the device has the proper training to perform the procedure is noted, because considering the complexity of the patient subjected to HSCT, the surgeon's experience and skills are fundamental to success of implantation of the catheter. However, CVC care occurs not only during its surgical implantation, so the teaching of all professionals who handle the device are fundamental and effective measures that contribute to reduce rates of catheter-related infection21.
With respect to preparation of the skin, evidence suggests sanitization followed by application of a similar alcohol solution10, a procedure that was performed in this study in 63 (81.8%) cases. Furthermore, chlorhexidine has greater residual effect than other antiseptic agents22, and was used in 96.1% of the procedures. As noted, most of the procedures were done with local anesthesia, which should be performed with 1% xylocaine without adrenaline10.
By adopting some preventive measures such as performing the procedure in the operating room and using aseptic techniques in skin preparation, the team contributes significantly to control infection2. Thus, it should be noted that the results of this study demonstrate such concerns on the part of the professionals who performed the surgical procedures.
The right internal jugular vein was the main venous access used, corresponding to 58 (75.3%) cases. Complications during catheter implantation occurred in four cases, two of which were due to bad positioning, one hemothorax, and one related to problems with the guide wire.
The internal jugular vein as the main choice of place for puncture is due to its rectilinear passage and surface location, which reduces the risk of bad positioning and, consequently, manipulation of the place and duration of the procedure10. On the other hand, some scholars state that the insertion site of the catheter influences the risk of infection related to the device, and therefore the insertion should take place preferably in the subclavian vein, the second option being the jugular vein, and the last option being the femoral vein, because these last two are more conducive to development of infection23.
Early complications related to the catheter implant that can occur during or after surgical implantation include arrhythmia, bad positioning, arterial puncture, air embolism, pneumothorax, dehiscence, hemorrhage and damage to vessels and nerves21.
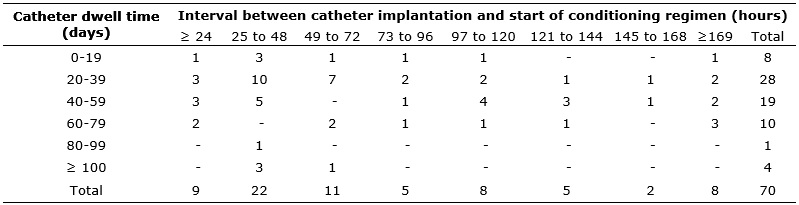
The interval between catheter implantation and start of the conditioning regimen was on average 5.1 days (varying from 8.5 hours to 36 days), and in 42 (60%) cases this interval was a maximum of 72 hours. It is noteworthy that 70 catheter implantations were considered, because one patient started the conditioning regimen with a catheter fully deployed, and the Hickman catheter was deployed later; four subjects had the first Hickman catheter taken out before start of the conditioning regimen; and in two cases it was necessary to replace the Hickman catheter after start of the conditioning regimen.
The mean stay time of the catheters was 42.8 days, given that one patient removed the catheter on the same day of the implantation, and the maximum dwell time was 118 days. The ratio of catheter dwell time and the interval between its implantation and start of the conditioning regimen are presented in Table 1.
TABLE 1: Distribution of dwell time of Hickman catheter relative to interval
between implantation and start of conditioning regimen. Ribeirão Preto, São
Paulo, 2006-2010 
It is recommended that chemotherapeutic agents are not administered less than seven days after catheter implantation, in order to prevent impairment of healing18. As regards the use of busulfan, the interval above is important because it is a dermatologically toxic agent that, in addition to causing adverse effects such as dermatitis and erythema, influence the healing process19. However, this study identified that the conditioning regimen was initiated on average five days after implantation of the catheter, which could interfere with the healing process and thereby increase the incidence of complications, particularly infectious, related to use of the CVC.
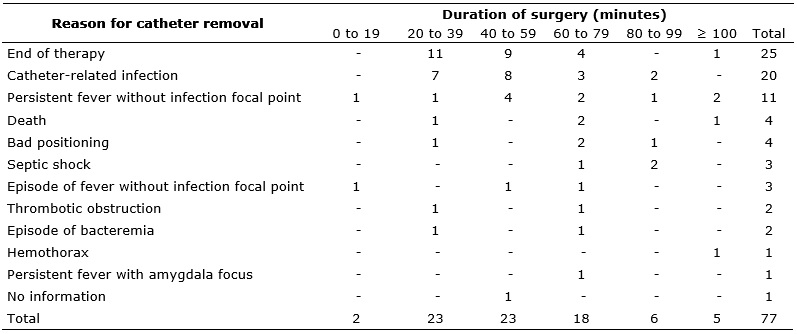
Among the 64 subjects that had between 21 and 79 minutes of operating time, 24 (37.5%) withdrew the catheter due to the end of therapy, and 18 (28.1 percent) due to catheter-related infection. It is noteworthy that the therapy was considered to be ended when the catheter was removed without record of a reason and unrelated to any other cause. One study presented as possible explanation for the short dwell time of Hickman catheters the medical team's concern to remove the device after completion of clinical therapy in order to prevent possible late complications, even if it was a long dwell time13. The reasons for removal of Hickman catheters, according to the duration of their respective surgical implantations, are presented in Table 2.
TABLE 2:
Distribution of reasons for removing Hickman catheters according to
duration of surgical implantation. Ribeirão Preto, São Paulo, 2006-2010
Catheter-related infections were confirmed in 26 (33.7%) of the 77 implantations performed. Of this total, 12 (46.2%) were characterized as a bloodstream infection; 8 (30.8%) as infection of the subcutaneous tunnel; 4 (15.4%) as infection of the catheter exit site; 1 (3.8%) as infection of the exit site and bloodstream; and 1 (3.8%) as infection of the catheter tunnel and bloodstream. Of all cases of infection, 9 (34.6%) were characterized as surgical site infection, with onset of signs and symptoms within 30 days after implantation of the catheter.
In this study, completion of therapy was the main reason for removal of 25 (32.4%) catheters, followed by occurrence of infection in 20 (25.9%) cases. These results corroborate the findings of other studies, in which infection was the most frequent complication associated with removal of the Hickman catheter5,12.
One study developed in three units with patients undergoing allogeneic HSCT pointed out that most removals of the first Hickman catheter occurred within 60 days after its implantation, with a mean dwell time of 45.1 days 5, similar to the data presented in this study (mean = 42.8 days).
Another study showed that the time of neutropenia prior to grafting of the SCT is a predictive factor for occurrence of infection24. Other authors argue that neutropenia is one of the most troubling phases, during which measures to prevent infection are essential to a favorable treatment outcome25.
It is estimated that bloodstream infection represents 10% to 15% of all hospital-acquired infections. Despite not being the hospital infection with the highest rate, this contributes to extension of hospital length of stay, causing significant burden to health institutions26, in addition to obvious harm to patients, since infection in recipients of HSCT is related to high rates of morbidity and mortality27.
Therefore, the need to prevent infections through standardization of care related to the insertion, manipulation and maintenance of the device is clear, in view of the importance of this technology for continuity of patient treatment.
Given the above, it is understood that the process of HSCT involves highly complex actions and requires a specialized and qualified multidisciplinary team to assist the patient and family in all stages of the process28. Although the nursing staff is closest to the handling of the CVC, and, consequently, with important possibility of activity on prophylaxis and control of related infections, it is emphasized that care with procedures that involve vascular access must be a priority of the entire care team.14
In addition, in order for the therapy to be successful, continuous efforts, with a focus on professional training and continuing education among all the professionals involved in patient care, must be invested in14.
CONCLUSION
This study described factors involved in surgical implantation of the Hickman catheter. The surgeries had a mean duration longer than that recommended by the literature, and the conditioning regimen was initiated earlier than recommended. Catheter-related complications were the main reasons for removal of the device, especially for infections.
Such results suggest that the start of the conditioning regimen earlier that recommended may contribute to the development of complications that lead to their withdrawal. Thus, the importance of nursing staff having specific knowledge and skills for the early identification of potential complications related to the catheter, in order to propose effective interventions to ensure the safety of the device and keep it free of complications that aggravate the already compromised health of this public, is fundamental.
The authors emphasize the need for further clinical studies on surgical implantation of Hickman catheters and occurrence of infections. The absence of information recorded in patients' medical records was one of the limitations of this study.
REFERENCES
1.Bochi KCG, Kalink LP, Camargo JFC. Assistência de enfermagem em transplante de células-tronco hematopoiéticas alogênico: cuidados baseados em evidências. Prática hospitalar. 2007:31-7.
2.Neves Junior MA, Melo RC, Goes Junior AMO, Protta TR, Almeida CC, Fernandes AR, et al. Infection of long-term central venous catheters: review of the literature. J vasc Bras. 2010; 9:46-50.
3.Albuquerque MP. Cirurgia dos cateteres de longa permanência (CLP) nos centros de transplante de medula óssea. Medicina. 2005; 38:125-42.
4.Santos KB, Rodrigues AB. Prevention of complications of the central venous catheter in bone marrow transplantation. Rev Min Enferm. 2008;12:119-26.
5.Castanho LC, Silveira RCCP, Braga FTM, Canini SRMS, Reis PED, Voltarelli JC. Rationale for Hickman catheter removal in patients undergoing hematopoietic stem cell transplantation. Acta Paul enferm. 2011; 24:244-8.
6. Bonassa EMA, Gato MIR. Terapêutica oncológica para enfermeiros e farmacêuticos. Rio de Janeiro: Atheneu; 2012.
7. Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat rev immunol. 2012; 12:403-16.
8. Hickman RO, Buckner CD, Clift RA, Sanders JE, Stewart P, Thomas ED. A modified right atrial catheter for access to the venous system in marrow transplant recipients. Surg Gynecol Obstet. 1979; 148: 871-5.
9.Silveira RCCP, Braga FTMM, Garbin LM, Galvão CM. The use of polyurethane transparent film in indwelling central venous catheter. Rev Latino-Am Enfermagem. 2010; 18:1-9.
10.Yasbek G, Nishinari K, Natal SRB. Técnicas de inserção por punção. In: Wolosker N, Kuzniec S. Acessos vasculares para quimioterapia e hemodiálise. São Paulo: Atheneu; 2007. p. 31-8.
11.Patel PM, Thakkar JM, Patel BM. Long term venous access with Hickman catheter in cancer patients: our initial experience at G.C.R.I. Gujarat Medical Journal. 2010; 65(1):30-4.
12.Pereira JZAP, Braga FRMM, Garbin LM, Castanho LC, Silveira RCCP. Permanência do cateter de Hickman em pacientes submetidos a transplante de células-tronco hematopoéticas alogênico: estudo retrospectivo. Rev bras cancerol. 2013; 59:539-46.
13.Castagnola E, Molinari AC, Giacchino M, Chiapello N, Moroni C, Caviglia I, et al. Incidence of catheter-related infections within 30 days from insertion of hickman – broviac catheters. Pediatr blood cancer. 2007;48:35-8
14.Mendonça KM, Neves HCC, Barbosa DFS, Souza ACS, Tipple AFV, Prado MA. Nursing care in the prevention and control of catheter-related Bloodstream infections. Rev enferm UERJ. 2011;19:330-3.
15.Galvão CM, Sawada NO, Rossi LA. A prática baseada em evidências: considerações teóricas para sua implementação na enfermagem perioperatória. Rev Latino-Am Enfermagem. 2002;10:690-5.
16.Craddock CF. Full-intensity and reduced-intensity allogeneic stem cell transplantation in AML. Bone marrow transplant. 2008;41:1-9.
17.Passweg JR, Baldomero H, Bregni M, Cesaro S, Dreger P, Duarte RF, et al. Hematopoietic SCT in Europe: data and trends in 2011. Bone marrow transplant. 2013; 48:1161-7.
18.Payne WG, Naidu DK, Wheeler CK, Barkoe D, Mentis M, Salas RE, et al. Wound healing in patients with cancer. Eplasty. 2008;8:68-90.
19.Benhamou E, Fessard E, Com-Nougué C, Beaussier PS, Nitenberg G, Tancrède C, et al. Less frequent catheter dressing changes decrease local cutaneous toxicity of high-dose chemotherapy in children, without increasing the rate of catheter-related infections: results of a randomized trial. Bone marrow transplant. 2001; 29:653-8.
20.O'Grady N, Alexander M, Burns LA, Dellinger P, Garland J, Heard SO, et al. Summary of recommendations: guidelines for the prevention of intravascular catheter-related infections. Clin infect dis. 2011; 52:1087-99.
21.Schiffer CA, Mangu PB, Wade JC, Camp-Sorrell D, Cope DG, El-Rayes BF, et al. Central venous catheter care for the patient with cancer: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2013; 31:1357-70.
22.Bashir MH, Olson LKM, Walters S. Suppression of regrowth of normal skin flora under chlorhexidine gluconate dressings applied to chlorhexidine gluconate-prepped skin. Am J infect control. 2012; 40:344-8.
23.Couto RC, Pedrosa TMG, Nogueira JM. Infecção hospitalar e outras complicações não-infecciosas da doença: Epidemiologia, controle e tratamento. 4ª ed. Rio de Janeiro: Guanabara Koogan; 2009.
24.Nieboer P, de Vries EG, Mulder NH, Rodenhuis S, Bontenbal M, Van der Wall E, et al. Factors influencing catheter-related infections in the Dutch multicenter study on high-dose chemotherapy followed by peripheral SCT in high-risk breast cancer patients. Bone marrow transplant. 2008; 42:475-81.
25.Garbin LM, Silveira RCCP, Braga FTMM, Carvalho EC. Medidas utilizadas na prevenção de infecções em transplante de células-tronco hematopoéticas: evidências para a prática. Rev Latino-Am Enfermagem. 2011; 19(3):[12 telas].
26.Pedrolo E, Danski MTRD, Mingorance P, Lazzari LSM, Johann DA. Ensaio clínico controlado sobre o curativo de cateter venoso central. Acta Paul Enferm. 2011;24:278-83.
27.Garnica M, Machado C, Cappellano P, Carvalho VVH, Nicolato A, Cunha CA, et al. Recomendações no manejo das complicações infecciosas no transplante de células-tronco hematopoéticas. Rev Bras Hematol Hemoter. 2010;32(Supl. 1):140-62.
28.Mercês NNA, Erdmann AL. Enfermagem em transplante de células tronco hematopoéticas: produção científica de 1997 a 2007. Acta Paul Enferm. 2010;23:271-7.