ORIGINAL RESEARCH
Microbiological evaluation of surfaces in intensive care: thinking about nosocomial infection prevention strategies
Adriana Costa GilI, Ana Paula Pegado BordignonII, Eduardo Almeida Ribeiro de CastroIII, Silvia Thees CastroIV, Ricardo de Mattos Russo RafaelV, José Augusto Adler PereiraVI
I
Nurse. Nursing School of the University of Rio de Janeiro State. Rio de
Janeiro, Brazil. E-mail: adrianagil.enf@gmail.com
II
Nursing Student. Nursing School of the University of Rio de Janeiro State.
Rio de Janeiro, Brazil. E-mail: bordignonanaenf@gmail.com
III
Doctor. Master in Collective Health. Member of the Hospital Infection
Control Committee. University Hospital Pedro Ernesto. Rio de Janeiro,
Brazil. E-mail: eduardorcastro@ig.com.br
IV
Doctor. Specialist in Infectious Diseases. Member of the Hospital Infection
Control Commission. University Hospital Pedro Ernesto. Rio de Janeiro,
Brazil. E-mail: stheescastro@gmail.com
V
Nurse. Ph.D. in Science. Adjunct Professor, Nursing School. University of
Rio de Janeiro State. Brazil. E-mail:
prof.ricardomattos@gmail.com
VI
Doctor. Ph.D. in Science. Associate Professor, Medical School. University
of Rio de Janeiro State. Brazil. E-mail: jadlerpereira@gmail.com
DOI: http://dx.doi.org/10.12957/reuerj.2018.26388
ABSTRACT
Objective: to determine the microbiological profile of bacteria isolated and identified from beds and infusion pumps in the intensive care unit of a university hospital in Rio de Janeiro state. Method: nine samples were collected from patients' bed side rails and eight from infusion pump keypads in an intensive care unit in October 2014. An area of 100cm² was delimited as the sampling parameter. Samples were collected using sterile swabs, which were wetted and transported with Cary-Blair. The microorganisms were isolated, classified, and then tested for antimicrobial resistance. Results: coagulase-negative Staphylococcus was the most prevalent type. Antimicrobial susceptibility testing indicated some of these Staphylococci were multi-drug resistant. Conclusion: multi-professional discussion of hospital safety issues must be expanded, and continuing professional development emerges as one possible pathway to success in nosocomial infection control.
Descriptors: Cross infection; bacteria; intensive care units; nursing.
INTRODUCTION
Health Care Related Infections (IRAS) can be defined as infections acquired by the individual through healthcare - diagnostic or therapeutic procedures - performed by health professionals, whether in a hospital, outpatient or even home environment. Although it is a much-debated subject, it still becomes relevant to express it as an indicator of quality of care, contemplating the safety of the patient and health professionals. IRAS can work as good quality markers, since 5 to 10% of patients who use hospital services acquire infection during hospitalization1,2.
IRAS can originate in two ways: endogenous and exogenous. The endogenous comes from the patient's microbiota, that is, through the imbalance of the immune system the microbiota can develop the infectious process. The exogenous IRAS is caused by agents external to the individual and can be transmitted by health professionals or by hospital objects. In this case, during the period of hospitalization, the patient acquires the microorganisms from the environment, becoming part of their own microbiota flora. If there is an imbalance in the patient's immune system, the microorganisms constituting the microbiota may lead to an infection2.
Some factors are predisposing to the establishment of IRAS, such as age extremes (newborns or old people), obesity, malnutrition, immune-depressive disease, and the use of some medications and smoking. Besides these previously mentioned factors, there are others that contribute to the acquisition of infections, such as prolonged hospitalization time, the need for invasive procedures and the indiscriminate use of antimicrobials, which can cause imbalance of the immune system and favor the appearance of these infections. IRAS are quite frequent and increasingly present in hospitals, and the patients of the Intensive Care Unit (ICU) are the most susceptible because they have a weakened defense mechanism and exposure to a greater number of invasive procedures3,4.
In these scenarios, there is evidence that several "classical" and opportunistic pathogens contaminate surfaces and equipment (infusion pumps, bedsheets, stethoscopes, etc.) more frequently handled by healthcare professionals such as methicillin-resistant Staphylococcus aureus,Enterococcus resistant to vancomycin, Pseudomonas aeruginosa, Acinetobacter baumanni and other potential agents of infections. Surface-related infections demonstrate an imminent risk to patient safety in the hospital setting. Failures in the processes of cleaning and disinfection of surfaces may result in the dissemination and transfer of microorganisms in the health service environments5.
Surface cleaning and disinfection are important to bring the sense of well-being, safety and comfort to patients, family members, and health professionals, also contributing to the control of IRAS, leading to a reduction in the number of microorganisms, areas appropriate to the practice of activities related to health services. The environment is considered an important reservoir of microorganisms in these services, especially multiresistant, and also can accumulate organic matter, favoring the proliferation of microorganisms5.
From a perspective of controlling these infections, nurses are considered as important agents of the process, being able to act in the control and prevention through direct actions and also in the management of the procedures executed by the team. They can also act as facilitators in the permanent education team, so there is adequacy and safety of the procedures performed. The nurse has been identified as one of the most important integrating agents of the multi-professional team, acting in the patient care in a holistic and humanized manner, in which it reflects not only in the control of IRAS but also in the care given to the individual6.
However, there are still few studies in the nursing area that focus on the isolation of microorganisms and antimicrobial susceptibility profiles, perhaps due to the high variability among hospital units. It is believed that these reflections are essential, from the formation to the daily practice of this professional, for guiding the programming of control actions by the team, especially by nurses.
In this sense, this study aims to determine the microbiological profile of bacteria isolated and identified in beds and infusion pumps in the intensive care unit of a university hospital in the State of Rio de Janeiro.
LITERATURE REVIEW
Multiresistant bacteria have been a matter of great concern, mainly because of the high involvement in the development of hospital infections. Those with the highest incidence are coagulase-negative Staphylococcus, Staphylococcus aureus,Acinetobacter baumanni, Pseudomonasaeruginosa, Klebsiella pneumonia, among others6,7.
For coagulase-negative Staphylococcus (SNA), although they are constituents of the resident microbiota of the skin, colonization in invasive devices has been reported with some frequency, making it a problem of great clinical importance, and being present in the most diverse hospital-level infections. There are several species of SCN,S. epidermidis as most commonly. It is also known that the Staphylococcus is mostly resistant to oxacillin, hindering to conduct them through drug therapy in the face of IRAS8.
SCNs have been identified as opportunistic agents that cause nosocomial and community infections. Among the most frequent there are S. haemolyticus and S lugdunensis, which have already been found in catheters of ICU neonates, causing otitis, urinary tract infections, osteomyelitis, endocarditis and even septicemia9,10. Infections caused by SCN show a major public health problem, due to the increase in the occurrence of bacterial resistance11.
Although restricted to the hospital environment, Klebsiella pneumoniae is another important bacterium. Its multidrug-resistant form is often related to the indiscriminate use of antibiotics, such as the third generation cephalosporins: ceftriaxone, cefotaxime and ceftazidime; and associated with horizontal transmission among patients7,10. It is noteworthy that Klebsiella pneumoniae is an opportunistic microorganism in immune-compromised patients, especially those in ICUs6,7.
Escherichia coli , Pseudomonas aeruginosa, and Acinetobacterbaumannii are also pathogens of clinical importance. Escherichia coli is a bacterium of the resident microbiota of the gastrointestinal tract. However, its incidence in infections is quite common. Frequently found in the urinary tract of patients, it has been reported as one of the main agents of infections related to the use of bladder catheter of delay in the intensive care environment6.
P. aeruginosa can cause severe infections of the lower airways, ranging from benign colonization or tracheobronchitis to severe necrotizing bronchopneumonia. Colonization is observed in patients with cystic fibrosis, other chronic lung diseases, or neutropenia. However, Pseudomonas infections are usually opportunistic, that is, they develop in immune-compromised patients12.
Acinetobacter baumannii is the most frequent pathogen in ICU, colonizing patients and the health team. It is associated with hospital infections, such as septicemia, meningitis and, predominantly, pneumonia. Transmitted primarily through cross-infection, they may be of great importance in the hospital environment because they survive on wet and dry surfaces and especially for colonizing the patients´ skin, facilitating their dissemination13. Regarding the prevention of IRAS and patient safety in the care setting, it is possible to highlight the hand hygiene in each performed procedure, besides care when performing invasive procedures14,15.
METHODOLOGY
This is a cross-sectional study combining laboratory test techniques for the isolation of microbial agents and antibiotic susceptibility to antimicrobials from each of them. The sample collection field was the ICU of a university hospital in the State of Rio de Janeiro, which provides care to patients of medium and high complexity in the secondary and tertiary levels, working with 525 beds. The ICU has 10 adult beds, where patients generally come from medical clinic wards. In the period studied, the bed occupancy rate was 100%.
The collection of biological material from the surfaces of infuser pumps and bed grids occurred in the period of October/2014. The selection of sample collection sites was based on the following criteria: site with no apparent dirt and, in the case of bed grids, the region closest to the patients was chosen. The selection of these surfaces occurred through the understanding that they would be sites close to the patient, but with different probabilities of manipulation, with the infusion pump being commonly handled by health professionals and bed grids by the general public. Patient surfaces were excluded in contact precaution since they would cause bias in the results of this study. The final sampling totaled 17 analyzed sites, being eight samples of infusion pumps and nine samples of bed grids.
The collection of biological material was carried out four hours after the disinfection of the surfaces with potassium monopersulfate. It is noteworthy that this disinfection was not controlled in this study, following the same standards used in the hospital. The collection procedure was performed by sterile swabs measuring 2x3cm in the following places: on the keyboard of the infusion pumps and on the bed grids, which followed the area standard of 10 x 10cm (100cm²).
After each sample collection, the respective swab was soaked in Carry & Blair transport buffer, which keeps the microorganism viable for longer because it contains nutrient formulation and absence of nitrogen, preventing the proliferation of the bacteria. The 18 x 10 cm tubes were stored in a thermal box containing six reusable ice packs, used to store samples at low temperature. After arriving at the laboratory, approximately 3 hours, the samples were seeded in sheep's blood agar and kept for 24 hours in a greenhouse at 36° in the laboratory of the Department of Microbiology and Immunology (DIMI), located at the Medical Sciences School, University of State of Rio de Janeiro (UERJ).
After 24 hours of stay in the greenhouse, the presence of microorganisms growth was evaluated and then the morphotintorial analysis was performed when bacterial colonies were growing in them, using standard biochemistry tests and complements for gram-positive and negative microorganisms for bacterial identification, described in the microbiological identification manual of the National Agency of Sanitary Surveillance (ANVISA)10. The results of the biochemical tests were interpreted through the tables of the Illustrated Atlas Koneman's12 and the Manual of Clinical Microbiology of ANVISA10. At the end of the identification of the microorganisms, the samples were submitted to antimicrobial susceptibility tests (TSA) standardized by ANVISA16, determining the sensitivity of the microorganisms to the antimicrobials.
The antimicrobial susceptibility profile was determined using CLSI 201517, using the following substances: Piperacyclin + Tazobactam (10μg), Cephalotin (30μg), Cefotaxime (30μg), Ceftriaxone (30μg), Chlorphenicol (30μg), Cotrimoxazole, Ampicillin + Sulbactam (10μg), Tetracycline (10μg), Neomycin Ampicillin (10μg), Ampicillin (10μg), Amoxicillin (20μg), Penicillin G (10 units), Ertapenem (10 μg), Tobramycin (10μg), Gentamycin (10μg) (SENSIFAR), Nitrofurantoin (300μg), Imipenem (10μg), Cefepima (30μg), Kanamycin (30μg), Norfloxacin (10μg), Cefuroxima (30μg), Meropenem (10μg), Rifampicin (5μg), Ceftazidime (30μg), Sulphamethoxazole-Trimetropim (25μg), Ciprofloxacin (5μg), Oxacillin (1μg), Erythromycin (15μg) and Clindamycin (2μg) (OXOID). In the antimicrobial susceptibility tests (TSA) bacterial suspensions were obtained from bacterial colony, cultured in 24 h at 35ºC, in 0.9% saline, with turbidity corresponding to grade 0.5 of the McFarland scale. We consider antimicrobial multiresistant samples resistant to three or more different classes of antimicrobials.
In the descriptive phase of the analysis, a database containing the species and amount of microorganisms observed as well as the collection site were constructed in the Microsoft Excel software. The treatment and statistical processing of the results occurred by a technique of univariate analysis, estimating the proportions of surfaces colonized by each type of agent. As a study that did not involve human beings, this work was exempted from submission and appreciation by the Research Ethics Committee.
RESULTS AND DISCUSSION
The proportion of sites with the presence of microorganisms and the discrimination of the identified species are shown in Table 1.
TABLE 1: Proportion of infusion pump surfaces (n=8) and bed grids (n=9)
with presence of bacterial species in the Intensive Care Unit. University
Hospital of the State of Rio de Janeiro, 2014-2015. 
The colonization of S. haemolyticus and S. hominis grading sites with 33.3% are highlighted, followed by S. simulans 22.2%. The pumps were 62.5% of S. haemolyticus, 37.5 of S. hominis and finally 25% of S. saprophyticus.
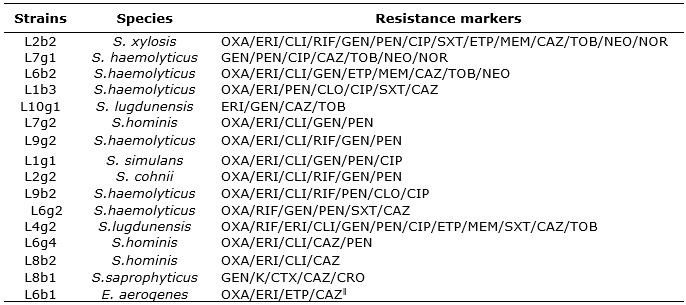
The results of the antibiogram tests are specified in Table 2.
TABLE 2:
Profile of multiresistant microorganisms on infusion pump surfaces and bed
grids in the ICU. University Hospital of the State of Rio de Janeiro,
2014-2015.

Legend: OXA: oxacillin; ERI: erythromycin, CLI: clindamycin, RIF:
rifampicin; GEN: gentamicin; PEN: penicillin; CIP: ciprofloxacin; SXT:
sulfamethoxazole + trimethoprim; ETP: ertapenem; MEM: meropenem; CAZ:
ceftazid; TOB: tobramycin; NEO: neomycin; NOR: norfloxacin; CLO:
clorafenicol; K: kanamycin; CTX: cefotaxime; CRO: ceftriaxone; TET =
tetracycline.
According to the sensitivity data presented, the microorganismsS. hominis, S. haemolyticus, S. simulans, S.lugdunensis among others, presented as multiresistant. Analyzing the results of the antimicrobial susceptibility test (TSA), a marked resistance of coagulase-negative Staphylococcus (SNA) to oxacillin was observed. The E. aerogenes pathogen in the TSA, found in the unit's infusion pump, proved to be multi-resistant to antimicrobials. However, some S. hominis, S. haemolyticus, S. saprophytcus, and S. simulans were also susceptible to antimicrobials.
The option of conducting the microbiological evaluation of surfaces allowed greater reflection on the risks of infection attributed to patients hospitalized in the study scenario. Surfaces, in general, are not valued as potential sources of transmission of inpatient infection agents. However, it is important to consider that the presence of organic matter that frequently contaminates these surfaces acts as a nutrient in these types of reservoirs of microbes with pathogenic potential, increasing the need to implement preventive actions that contemplate these sites18.
For the different surfaces analyzed, the genus Staphylococcus spp was found to be the most prevalent - both infusion pump and bed grids. It can also be recognized that bacteria such as S. hominis, S. haemolyticus, and S. simulans were more frequent in infusion pumps. As observed in other studies, the frequency of Staphylococcus was also quite pronounced on these surfaces, with possible explanation of the frequent handling of this type of equipment 19-21. These Staphylococci are part of the normal microbiota of human skin. However, it has been reported the increasing number of infections caused by S. epidermidis, followed by S. hominis, S. haemolyticus, and S. capitis, most often multiresistant to antimicrobials20.
Among the species found, S. haemolyticus is highlighted and it has been associated with IRAS in immune-compromised patients19. It is considered that such finding may be compatible with the results of this study since the patients hospitalized in the scenarios surveyed commonly have some degree of impairment of the immune system. Research on intensive care patients has pointed to S. haemolyticus as an important cause of bloodstream infections associated with the use of central venous catheters. The agent has also been reported as one of the causes of septicemia in neonatal ICU in newborn infants who use peripheral venous catheters9-12. Among the explanatory hypotheses for the occurrence of this phenomenon is in the very frequency of use intravenous devices, which facilitate the proliferation and entry of the microorganisms into the bloodstream.
Over time, the microorganisms have developed mechanisms of resistance to various antimicrobials. Through the action of antimicrobials or even the existence of structures and mechanisms, they may inhibit the action of drugs1.
According to recent studies, a high prevalence of oxacillin (methicillin) resistant SCN occurs. This resistance is highly demonstrated by the mec A gene. This gene is responsible for the synthesis of PBP2a (Penicillin-binding Protein 2a). This gene is part of a genomic resistance island called SCCmec (Staphylococcal Cassette Chromosome mec), which is currently classified into 11 types11. These regions may also contain other antimicrobial resistance genes. Bacterial resistance can be developed through transformations in the genetic code, mutations or gene transfer between bacteria. These modifications can be expressed by making the microorganisms progressively resistant to antimicrobials, hindering or even rendering the therapy unfeasible. The causes of this process are associated to the indiscriminate, empirical and daily prescription of antimicrobials in clinical practice.
Bacterial resistance can lead to several implications in the hospital environment, such as increased hospitalization time, impaired treatment of certain diseases, limited choice of drugs and even the need to introduce new drugs into clinical practice for control of infections. Moreover, the use of broad-spectrum drugs in daily practice facilitates this process of resistance, since they should be part of the last choice therapy.
The fact that these multi-resistant opportunistic microorganisms probably are not community is highlighted, but they are part of the hospital microbiome and are circulating in hospitals in critical times. Usually, infusion pumps are handled in a restricted way by health professionals who inevitably ends up gaining prominence in this work.
Hand cleaning is essential to reduce infections in critical areas, as well as to prevent aggravations of established infections. Taking as reference the norms of the National Health Surveillance Agency, hand hygiene should comply with five moments: before contact with the patient, before the aseptic procedure, after exposure to the risk of contact with body fluids, after contact with the patient and after contact with areas close to the patient22.
Also, following the recommendations of ANVISA, the handling of equipment, as in the case of infusion pumps, should be preceded by the use of personal protective equipment, such as gloves23. In this sense, the microorganisms found in the pumps are especially highlighted by the possibility of having been transmitted through the hands of professionals, either by manipulation of previously contaminated surface or even by inadequate hand hygiene.
Differently, from what was assumed, the bed grids presented smaller amounts of microorganisms, even though the surfaces were probably more exposed and contaminated by the patient's own handling, relatives, and professionals involved in direct care. It is believed that this is due to the results of adherence to the various recommendations on hand hygiene, by washing with soap and water or using 70% alcohol by nursing professionals. It is important to consider that in many services, the very entry of people into intensive care settings is conditioned to this practice, a point that needs a study to analyze the results over time.
According to the Ministry of Health, the use of alcohol is recommended due to its bactericidal action, and in case of non-use, water, and liquid soap are used24,25. It is important that these hygienic measures be adopted for each procedure performed and from one bed to another, as well as changing the gloves whenever necessary1,25.
Failures resulting from the cleaning and disinfection of surfaces can also lead to the presence of these bacteria on the surfaces examined. A recent study showed that even in the face of the knowledge about the importance of these procedures, adherence to the technique of hand hygiene is still low, especially related to work overload and lack of environmental conditions, such as the provision of materials and equipment needed in strategic locations26. Another aspect that deserves reflection is the contamination during alcoholic preparation for hand hygiene, which can hinder the procedure and increase contamination of surfaces27.
Therefore, it is necessary to supervise a trained professional during the execution of these procedures, to clarify the doubts in the cleaning and disinfection and also the training of the employees so the sanitizing products are applied correctly.
The nurse of the Hospital Infection Control Commission has the task of supervising the hospital sectors to prevent and control infections and to provide up-to-date information on the subject so they can be taken to the health teams by the nurse responsible for each sector24. In view of the results found, it is possible to point out the need for permanent education to control infections, not only with a focus on hand hygiene, but also on the use of protective equipment, cleaning, and disinfection of surfaces, and especially on need of reflection of the processes that involve the work. It is important to constantly consider the need for approaches that address IRAS and patient safety, aiming at greater adherence to infection prevention measures, making the care qualified for the patient and the professional.
CONCLUSION
The results presented should be interpreted based on their limitations. The first limitation is the possibility of the presence of microorganisms before the disinfection procedures since there was no collection that allowed comparisons before and after the process. With this, it is important to emphasize that the conclusions presented here deal with possible explanatory hypotheses. The second limitation is that it is not possible to generalize the results to other scenarios without first checking the characteristics of comparability, since the chosen unit is a university hospital and, for this reason, it has unique characteristics.
Even with these limitations, it is believed that the observation of the main agents involved in the contamination of hospital surfaces arouses, above all, the possibility of (re) thinking about practices directed to the control of IRAS and the necessary care with the infusion pumps and the grids of the beds. Identification of the genus Staphylococcus spp. as the most prevalent microorganism found in this study reinstates the debate on human skin microbiota as a potential reservoir of infection agents, especially when the pattern identified is multidrug resistance, as in the case of this work.
The differences in contamination between pump infusers and grids bring the reflection of the expansion of strategies that contribute to the awareness of health professionals regarding hand washing procedures and the correct use of protective equipment. It seems imperative to think of ways to build relationships capable of better thinking about the work process, with lifelong education being a possible way to achieve IRAS control more efficiently.
REFERENCES
1. Agência Nacional de Vigilância Sanitária. Manual of cleaning and disinfection of surfaces. Brasília: ANVISA; 2010.
2. Fernandes AT, Fernandes MOV, Ribeiro Filho N. Hospital infection and its interfaces in the health area. São Paulo: Ateneu; 2000.
3. Oliveira AC, Damasceno QS. Surface of the hospital environment as possible reservoirs of resistant bacteria: a review. Rev esc enferm USP [Internet]. 2010 [cited on 2017 Oct 12]; 44(4):1118-23. Available at: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0080-62342010000400038
4. Ferraz RRN, Lapchik MS, Barnabé AS, Fornari JV. Non-conformities in the precautionary/isolation practices and occurrence of Acinetobacter baumanii infections related to health care (IRAS) as an element of improvement in the management process. RASM [Internet]. 2014 [cited on 2017 Oct 12]; 4 (1): 19-29. Available at: http://www.saomarcos.br/ojs/index.php/rasm/article/view/58/56
5. Agência Nacional de Vigilância Sanitária (ANVISA). Ordinance nº 2.616, May 12th 1998. Establishing the guidelines and norms for the prevention and control of hospital infections. Official Diary of the Union, Brasília, DF, 13 mai. 1998 [cited on 2017 May 08]. Available at: http://bvsms.saude.gov.br/bvs/saudelegis/gm/1998/prt2616_12_05_1998.html
6. Oliveira AC, Paula AO, Iquiapaza RA, Lacerda ACS. Infections Related to Health Care and Clinical Severity in an Intensive Care Unit. Rev. gaúcha enfermagem [Internet]. 2012 [cited on 2017 May 10]; 33(3):89-96. Available at: http://www.scielo.br/pdf/rgenf/v33n3/12.pdf
7. Fonseca BO, Carvalho AFC, Gonçalves VD, Veloso RC, Alvarenga TF, Pereira AMS et al. Contamination of the hands of hospital staff with Enterobacteriaceae able to transfer resistance: an issue for hospital biosafety. Revista SODEBRAS [Internet]. 2014 [cited on 2017 March 10]; 9(107):81-85. Available at: https://www.researchgate.net/publication/274385238_Contamination_of_the_Hands_of_Hospital_Staff_with_Enterobacteriaceae_Able_to_Transfer_Resistance_An_issue_for_Hospital_Biosafety
8. Bezerra AB, Araújo EC, Alves WCL, Oliveira RA. Oxacillin resistant negative coagulase staphylococcus at the Araguaia Public Regional Hospital – Pará. Araguaia: Faculdade de Ensino Superior da Amazônia Reunida; 2010.
9. Pereira PMA, Binatti VB, Sued BPR, Ramos JN, Peixoto RS, Simões C et al. Staphylococcus haemolyticus disseminated among neonates with bacteremia in a neonatal intensive care unit in Rio de Janeiro, Brazil. Diagnostic Microbiology and Infectious Disease [Internet]. 2014 [cited 2017 Mar 22]; 78(1):85-92. Available at: https://www.sciencedirect.com/science/article/pii/S0732889313003829
10.Agência Nacional de Vigilância Sanitária. Clinical microbiology for the control of infection related to health care. Brasília: ANVISA; 2013.
11.Sued BPR. Staphylococcus haemolyticus and Staphylococcus epidermidis isolated from hospital sources: profiles of resistance to antimicrobial agents and biofilm production [master's dissertation]. Rio de Janeiro: Universidade do Estado do Rio de Janeiro; 2014.
12.Winn WCB, Allen SD, Janda WM, Koneman EW, Schreckenberger PC, Procop G, et al. Microbiological diagnosis: text and colored atlas. 6ª ed. Rio de Janeiro: Guanabara Koogan; 2008.
13.Moraes GM, Cohrs FM, Assayag BRE, Satovschi GR. Infection or colonization by resistant microorganisms: identification of predictors. Acta paul. enferm. [Internet]. 2013 [cited on 2017 April 20]; 26(2):185-91. Available at: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-21002013000200013&lng=en
14. Santana RS, Brito BAM, Ferreira JLS, Deus SRM, Moraes MEA, Gama MEA. Nurse assignment in the Hospital Infection Control Commission: Integrative review. Rev Pre Infec e Saúde [Internet]. 2015 [cited on 2017 April 04]; 1(2):67-75. Available at: http://www.ojs.ufpi.br/index.php/nupcis/article/view/4338
15.Murray PR. What Is new in clinical microbiology—microbial identification by MALDI-TOF Mass Spectrometry: a paper from the 2011 William Beaumont Hospital Symposium on Molecular Pathology . The Journal Of Molecular Diagnostic [Internet]. 2012 [cited 2017 Dec 13];14 (5):419–23. Available at: http://jmd.amjpathol.org/article/S1525-1578(12)00142-0/fulltext
16.Agência Nacional de Vigilância Sanitária (ANVISA). Standardization of antimicrobial susceptibility testing by disk-diffusion: approved standard. 8 ed. Brasília: ANVISA; 2015.
17.Clinical and Laboratory Standards Institute. Zone diameter Staphylococcus spp and Enterobacteriacea. New Jersey (USA): Clinical and Laboratory Standards Institute; 2015.
18.Becker AP, Cantareli VV, Rossato FCP, Inoue FM, Dias C, d'Azevedo PA. Non-Multidrug-Resistant, Methicillin-Resistant Staphylococcus aureus causing infection in health-care facilities in Southern Brazil. J Med Microb Diagn [Internet]. 2014 [cited 2017 Feb 2]; 3(3):150. Available at: https://www.omicsonline.org/open-access/nonmultidrugresistant-methicillinresistant-staphylococcus-aureus-causing-infection-in-healthcare-facilities-in-southern-brazil-2161-0703.1000150.pdf
19.Boretti VS, Corrêa RN, Santos SSF, Leão MVP, Gonçalves CR. Sensitivity profile of Staphylococcus spp. and Streptococcus spp. isolated from toys of a teaching hospital. Rev. Paul. Ped. [Internet] 2014 [cited on 2017 Feb 2]; 32(3):151-6. Available at: https://www.sciencedirect.com/science/article/pii/S0103058214700024
20.Moraes CL, Ribeiro NFG, Costa DM, Furlan VG, Palos MAP, Vasconcelos LSNOL. Contamination of equipment and surfaces of intensive care units by coagulase negative Staphylococcus from a public maternity hospital. Rev. patol. trop. [Internet] 2013 [cited on 2017 Nov 10]; 42(4):387-94. Available at: https://www.revistas.ufg.br/iptsp/article/viewFile/27927/15775
21.Silva PV, Cruz RS , Keim LS , Paula GR , Carvalho BT , Coelho LR , et al. The antimicrobial susceptibility, biofilm formation and genotypic profiles of Staphylococcus haemolyticus from bloodstream infections. Mem Inst Oswaldo Cruz [Internet]. 2013 [cited 2017 Mar 3]; 108(6):812-6. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0074-02762013000600812
22. Ministry of Health. Protocol for the practice of hand hygiene in health services. Brasília (DF): Ministry of Health; 2013.
23.Agência Nacional de Vigilância Sanitária. Manual for regularization of medical equipment in ANVISA. Brasília (DF): ANVISA; 2013.
24.Sales VM, Oliveira E, Célia R, Gonçalves FR, Melo CC. Microbiological analysis of inanimate surfaces of an Intensive Care Unit and patient safety. Rev. Enf. Referência [Internet]. 2014 [cited on 2017 April 5]; 4(3):45-53. Available at: https://rr.esenfc.pt/rr/index.php?module=rr&target=publicationDetails&pesquisa=an%E1lise%20microbiol%F3gica&id_artigo=2465
25.Agência Nacional de Vigilância Sanitária (ANVISA). Safe care: a theoretical reflection applied to practice. Brasília (DF): ANVISA; 2013.
26. Oliveira AC, Paula AO, Gama CS, Oliveira JR, Rodrigues CD. Adherence to hand hygiene among nursing technicians in a university hospital. Rev enferm UERJ [Internet]. 2016 [cited on 2017 April 20]; 24(2):e9945. Available at: http://www.facenf.uerj.br/v24n2/v24n2a15.pdf
27. Kusahara DM, Avelar AFM, Braga AV, Mendes MTM, Peterlini MAS, Pedreira MLG. Contamination of alcoholic preparation for hand hygiene in a pediatric intensive care unit. Rev enferm UERJ [Internet]. 2016 [cited on 2017 April 20]; 24(2):e10640. Available at: http://www.e-publicacoes.uerj.br/index.php/enfermagemuerj/article/view/10640/19418