ORIGINAL RESEARCH
Risk management in technovigilance: analysis of reports from a sentinel hospital
Renata Sodré SousaI; Leticia Prince Pereira Pontes II; Jacione Lemos Botelho MaiaIII; Hanna Arony Wandeley Pereira de AraújoIV; Tânia Pavão Oliveira Rocha V; Raquel Pereira DinizVI
I
Pharmacist. Resident in Child Healthcare. University Hospital of the
Universidade Federal do Maranhão. São Luís, Maranhão, Brazil. E-mail:
renata.sodresousa@gmail.com
II
Pharmacist. Master of Science in Health. Pharmacist of the Risk Management
Unit of the University Hospital of the Universidade Federal do Maranhão.
São Luís, Maranhão, Brazil. E-mail:
leticiaprince22@hotmail.com
III
Pharmacist. Doctorate student in Medical Sciences at the Universidade
Estadual do Rio de Janeiro. Pharmacist of the Risk Management Unit of the
University Hospital of the Universidade Federal do Maranhão. São Luís,
Maranhão, Brazil. E-mail:
jacionebotelho@superig.com.br
IV
Pharmacist. Master in Maternal and Child Health. Pharmacist of the Risk
Management Unit of the University Hospital of the Universidade Federal do
Maranhão. São Luís, Maranhão, Brazil. E-mail: hwpereira@gmail.com
V
Nurse. Master in Health Sciences. Nurse of the Risk Management Unit of the
University Hospital of the Universidade Federal do Maranhão. São Luís,
Marnhão, Brazil. E-mail: tpavãorocha@gmail.com
VI
Nurse. Specialist in Neonatology. Nurse of the Risk Management Unit of the
University Hospital of the Universidade Federal do Maranhão. São Luís,
Maranhão, Brazil. E-mail:
raquelpereiradiniz@gmail.com
DOI: http://dx.doi.org/10.12957/reuerj.2017.22730
ABSTRACT
Objective: to evaluate and analyze health product-related technical complaints and adverse events at Maranhão Federal University Hospital. Methods: from January to December 2015, 171 notifications were identified through the Health Surveillance Notification System and the Care Risk Management Unit data base. Results: most reports were technical complaints (90.06%) filed mainly by pharmacists (66.70%) and nurses (22.60%). Product fragility was the commonest type of quality deviation, with medium-risk products responsible for the most technical complaints, and gloves, the product most notified. Conclusion: educational strategies are thus necessary for professional improvement, to help improve care and implementation of a technovigilance risk mitigation plan to assure product quality without affecting the service being offered.
Keywords: Technology assessment biomedical; risk management; notice; patient safety.
INTRODUCTION
The prominent scientific and technological development made available to the health industrial complex promotes an accelerated insertion of new technologies in the market, sometimes without a systematic evaluation of their efficacy and safety1,2. Among other health problems resulting from the massive inclusion of these technologies are the technical complaints and the occurrence of incidents or adverse events that may cause harm to the health of users or health professionals involved in their operation, handling or application3.
All of this process led to a significant increase in health costs, with the production, acquisition and evaluation of new products, as well as investments in infrastructure and training of human resources1. It has been observed, however, that it was not considered that these new technologies would have intrinsic risks due to incomplete scientific knowledge about them, what they can generate and their interactions in different situations. The dissemination of these risks has induced pressure on governments to control them, requiring health surveillance to use inter-complementary health protection strategies4.
One of these strategies was the creation of the National Health Surveillance Agency (ANVISA), an agency of the Ministry of Health (MH), which aims at promoting and protecting the health of the population by sanitary control of the production and marketing of products and services, including the environments, processes, inputs and technologies in health 3,5,6. ANVISA is also responsible for coordinating the Sentinela Hospitals Network, which is based on three pillars: active search for adverse events, notification and rational use of health technologies, ensuring higher quality products that provide greater patient and health professionals safety7.
Also for this purpose, Hospital Risk Management (HRM) was set up in hospitals linked to the Sentinela Hospitals Network. The HRM develop post-marketing surveillance activities of health products through the National System of Notices in Sanitary Surveillance (NOTIVISA)8, in order to identify probable origins of adverse events, to evaluate the damages caused and to propose decisions concerning these problems 9.
In view of the above, the objective of this study was to evaluate and analyze technical complaints and adverse events related to health products, obtained through the Assistance Risk Management Unit (ARMU), in order to know the profile of medical article notifications in use in the University Hospital of the Universidade Federal of Maranhão (UHUFMA), and, consequently, to provide information in order to take measures and improve product quality and prevent event occurrences, ensuring the safety of patients and health service professionals.
LITERATURE REVIEW
A study conducted in some Latin American countries, between 2007 and 2009, has showed that 10.5% of hospitalized patients suffer from some type of adverse event, and of these, 58.9% could be avoided; results that were decisive for the culture of improving the quality and safety of the patient, besides knowing the magnitude of the problem10. To this end, the Pan American Health Organization, together with the World Health Organization, has indirectly promoted the development of adverse events notification systems through the process systematization, data collection and analysis11.
Therefore, to understand the extent of these data it is necessary to conceptualize some terms. An adverse event is the incident that resulted in injury or damage to a patient or professional, due to the use of a product subject to the sanitary surveillance regime, and its use has been performed under the conditions and parameters prescribed by the manufacturer 11,12. In contrast, a technical complaint is any notification of suspected alteration/irregularity of a product/company related to technical or legal aspects, and that may or may not cause harm to individual and collective health13.
Therefore, Techno-surveillance is understood as the system of surveillance of adverse events and technical complaints of health products in the post-marketing phase, with a view to recommending the adoption of measures that guarantee the protection and promotion of the health of the population 13. The notifications in this field become essential in obtaining data to measure the quality of the care provided, to provide subsidies for interventions, to provide changes in institutions and to ensure patient safety14.
Throughout the world it has been observed the need for research and records that can develop a data network, which facilitates the supervision and rapid feedback on the safety of the marketed medical products15, as an example we can mention the initiatives of the EU-ADR project of the European Commission16; in the United States of America, through the Food and Drug Administration (FDA), the Mini-Sentinel pilot program17; and the Observational Medical Outcomes Partnership 18. These projects manage to collect healthcare utilization records, covering millions of people. In Brazil, this supervision is carried out by ANVISA, which conceptualizes health products as materials or accessories whose use or application is linked to the defense and protection of individual or collective health, or for diagnostic and analytical purposes19.
This universe ranges from procedures gloves and condoms to surgical materials, prostheses, equipment and in vitro diagnostic kits, among others. Due to this wide variety and without ruling out that health products are capable of producing health problems and sequelae20 , ANVISA recommends the act of registration for the regularization of products21, and also the registration of the company itself with the agency. The company must also present evidence that proves the safety, quality and effectiveness of the product22.
At the time of registration of the product a classification that considers the risk that the product represents to the health of the consumer, patient, operator or third parties involved is proposed. According to this classification, the risk can be classified as: Low Risk - Class I; Medium Risk - Class II; High Risk - Class III and Maximum Risk - Class IV 23,24. After the registration, with large-scale production, the post-marketing surveillance process begins, where unexpected problems can be identified or an increase in unwanted events can be evidenced, highlighting the importance of Techno-surveillance in the health products regulatory process23.
One of the strategies used by Anvisa to encourage the notifications of products that are already on the market was the implementation of the Sentinela Hospitals Project in 2002, which was based on the creation and maintenance of a network of hospitals of great size and complexity, involved with assistance, teaching and research. This Network operates through Risk Management in all states of Brazil as an observatory of the behavior of health technologies, especially performance and safety issues 23. The purpose of this surveillance is to identify, prevent or minimize health problems related to the malfunction or manufacturing defects of these products, in order to instigate companies to raise their quality, which directly impacts on the quality of the products purchased, hospital cost, and the quality of care provided25.
METODOLOGY
It is a retrospective, cross-sectional and quantitative study addressing technical complaints and adverse events associated with health products. These notifications were made by the ARMO in the NOTIVISA system, during the period from January to December of 2015, in the UHUFMA.
The notifications were obtained online, through the NOTIVISA system, where the results of the research were filtered by period, which was from January 1 to December 31, 2015; by product of the notification, hospital medical article; and by types of notification, technical complaint and adverse event. From that point on, a list of 171 notifications was obtained, which was also included in ARMO internal control.
These notifications were evaluated according to the following variables: type of notification, which were arranged in a technical complaint or adverse event; professional notifier; the clinic with the highest number of reports; description of the deviation presented, which were grouped in fragility of the product, manufacturing defect, defects in labeling and packaging, and presence of foreign body; type of product notified; and classification of intrinsic risk.
In the NOTIVISA form, the description of the notification in Techno-surveillance is a subjective field, which, although contributing to the investigation and inspection actions, also makes it difficult to systematize the data. Therefore, for the categorization of reported technical complaints, the criteria suggested in the Vigilance Notification Book26 were used and in the most recurrent words in the descriptions of complaints. Four categories of technical complaint were constructed: product fragility, manufacturing defect, labeling and packaging, and foreign body presence. The fragility of the product category refers to problems of flexibility, breakage of the product or part of it, clogging, leakage and absorption. The manufacturing defect category is related to products with integral packaging that have cracks, holes, missing product or parts of it, improper size and shape, and malfunction. The category defects in labeling and packaging was constituted by problems in the identification of lots, validities and failure in the sealing of the packaging. Finally, the category presence of foreign body refers to any biological material or metals that are not inherent to the product.
The data has been organized and tabulated using the Microsoft Excel 2013 program. The results were expressed as average ± standard error of the averages (SEA) and presentations of the simple and percentage frequencies. The data has been evaluated using the Kruskal-Wallis non-parametric analysis, followed by the Dunns test using GraphPad Prism, Inc., Version 5.00.288 software, at a significance level of 5% (p <0.05).
The UHUFMA is formed by two large units of high complexity: Presidente Dutra and Mother Child. These units are intended to provide community healthcare through the Unified Health System (SUS), as well as teaching, research and extension in the health and related areas. It is an institution that is part of the Anvisa Sentinel Hospitals Network, which acts in the notification of adverse events and technical complaints in the areas of pharmaco-surveillance, techno-surveillance, haemo-surveillance and care areas.
RESULTS E DISCUSSION
In the period from January to December of 2015, 171 spontaneous and active search notifications were made related to the health products used in the hospital. Among these notifications, 17 (9.94%) adverse events related to medical-hospital articles were observed, compared to 154 (90.06%) notifications of technical complaints. A similar study also recorded an expressive majority of technical complaints related to hospital medical articles, possibly because they are simpler to evaluate and, most of the time, are visually perceptible20.
It is also believed that this percentage difference can be justified by the mistaken view that the notification of the adverse event is an accountability27 with a punitive character, and, therefore, it faces barriers like fear and guilt28, resulting in underreporting. It is, therefore, necessary to be aware that these events should be informed or notified to health service managers in order to be able to carry out risk management measures28. The act of notifying also contributes to the formation of a culture focused on patient safety, especially as it strengthens the trust of the organization in its employees29.
Regarding the professional category, pharmacists were the ones that made the most notifications, a total of 114 (66.70%), followed by nurses with 39 (22.60%). This study, as well as similar ones3,30,20, point out the pharmacist and the nurse as the professionals who most reported problems with health products. Nurses are more likely to report incidents because they spend more time with patients, are more likely to be in the hospital, and because of patient care, they have direct contact with the consumer and permanent supplies at the health facility28,20.
The results obtained can be explained by the training of the team that currently works in the Risk Management Unit of the hospital, which is essentially composed of pharmacists and nurses. Pharmacists stand out because, in this unit, the Pharmacist is directly responsible for the notifications in Techno-surveillance, through active search, which is an important method of identification and notification of occurrences at the hospital units30, or by evaluating spontaneous reports.
In relation to the several units of the service where the technical complaints occurred, it has been chosen by the grouping of some clinics and, thus, to promote a better visualization of the obtained data. The Neonatal, Pediatric, Adult and Cardiologic Intensive Care Units were generalized in the Intensive Care Unit (ICU) and the Children, Adult, Obstetric and Gynecological and Orthopedic Surgery Centers in Surgical Center.
The three clinics with the most reports were the ICUs, with 31 (18%), Surgical Center, 27 (16%), and Pediatrics, 24 (14%). It has also been observed that 17 (10%) notifications did not inform the place of origin.
The Surgical Center and ICUs are cited in some works3,7 as one of the places that most forward notifications. It is believed that this occurs because they are critical care areas that use a large volume of materials in different processes and that the use of sophisticated resources is inherent in the work process, which are essential in the development of activities. It is also possible to highlight that the professionals of these areas end up developing a more careful observation and evaluation capacity, as well as the valorization of the products 3,7. In contrast, a study pointed out that the knowledge about adverse events of the ICU professionals is superficial, even though they recognize the event as part of healthcare when it is not performed with quality31. Another factor that may justify the number of ICU notifications is the presence of the clinical pharmacist, who keeps an eye on health product conditions and maintains direct contact with the ARMO.
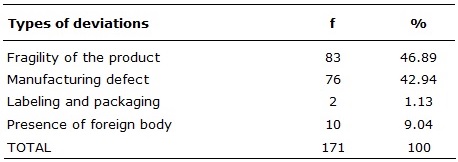
From these reports it was possible to identify the most recurrent types of deviations through the descriptions made of the occurrences. There were 83 (46.89%) Fragility Deviations of the product, followed by Manufacturing Defect with 76 (42.94%), Presence of Foreign Body with 10 (9.04%) and, finally, Labeling and Packaging with 2 (1.13%) of notifications.
TABLE 1:
Types of deviations presented in the medical-hospital products, Sentinel
Hospital in São Luís-Maranhão, January-December, 2015.

In each established category there are products that presented a distance of quality, not obeying some parameters required by ANVISA. This work is in agreement with another20, in which the same established categories have been adopted, presenting 17 (48.58%) notifications of deviations of the type Fragility of the product. In a similar study 7 a classification was made based on Anvisa criteria being categorized in packaging, structure and altered aspect. In this research, the Structure category stands out among the others, this refers to cracking, breaking of the product or part of it, problems related to fitting, obstruction, leakage, size, absorption, loss of cut, and presence of foreign body. Correlating these works it was possible to observe that deviations in the form and function of health products are common.
Hospital medical articles that present ruptures, ruptures or fragility may cause contamination and put at risk the safety of the health professional and the patient32. And for that reason its responsibility is shared between the company that holds the registration, which is responsible for the product from its design, use until the discard; and ANVISA, which is responsible for the sanitary control of the products at all stages of its life cycle33.
In relation to the most common product in the reports, the glove was the one with the highest frequency of technical complaints (25.99%), followed by the surgical tape 29 (16.38%) and syringe 18 (10.17%), the articles with less than five notifications were granted in the Others category.
TABLE 2
: Most medical articles notified by NOTIVISA. Sentinel Hospital, São
Luís-Maranhão, January-December/2015.
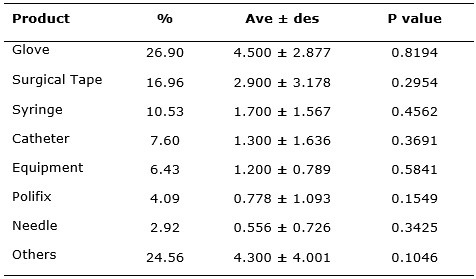
The data was expressed as percentage, average ± SEA (standard error of
averages) and P value, p <0.05, n=8.
The results indicate that the most notified products are widely used in health services and, therefore, there is a need to have a greater control over them. However, it is worth mentioning that within the Other category there are products that were responsible for serious adverse events that should never occur in health services (never events), related to product failures and/or devices used in healthcare34, that have been prioritized and established goals in which the deadlines and the investigative processes performed by the health services are being monitored.
Regarding the technical complaints about the surgical and procedural gloves grouped in this study as a single product, they are common factor in the national reality7. In order to improve the sanitary control over gloves, Anvisa created the resolution 55/2011 that establishes the minimum identity and quality requirements for surgical and non-surgical gloves used in the country. The resolution provides certification requirements for compliance under the Brazilian Conformity Assessment System (BCAS). With the exception of synthetic rubber and polyvinyl chloride gloves that shall only present a declaration at the time of application for the registration and revalidation that the company complies with the performance requirements set out in the new resolution35. The INMETRO Ordinance No. 332/2012 corroborates with the RDC, establishing requirements for the Glove Conformity Assessment Program through the compulsory certification mechanism of these products, with an exclusive focus on the health and safety of users36.
Another strategy that has been used to minimize the problems related to these products is the pre-qualification of the product prior to its acquisition. The prequalification can be defined as a legal, technical and functional assessment performed by a user who is generally relevant to the area that uses them the most, so a multiprofessional team is crucial to provide this technical and legal support to the acquisition of products. The prequalification of medical and hospital articles can contribute to the acquisition of articles that best meet the needs of the healthcare establishment, without considering the criterion of the lowest price37, because quality material can mean the survival of a patient 7.
Even in the face of these barriers, it is important to emphasize that there is no zero risk38 and that several factors can contribute to the inadequate performance of the product and to the occurrence of unfavorable outcomes, and, therefore, one can not rule out knowing the risks that are inherent in the product itself. This risk classification, proposed by Anvisa at the time of registration, contributes to a better analysis of the notifications and adequate monitoring of medical hospital articles in the market.
In this study, the Medium Risk products were responsible for 91 (59%) notifications of technical complaints, followed by 56 Low Risk (37%), 5 (3%) High Risk and 2 (2%) notifications were not classified by the NOTIVAL system.
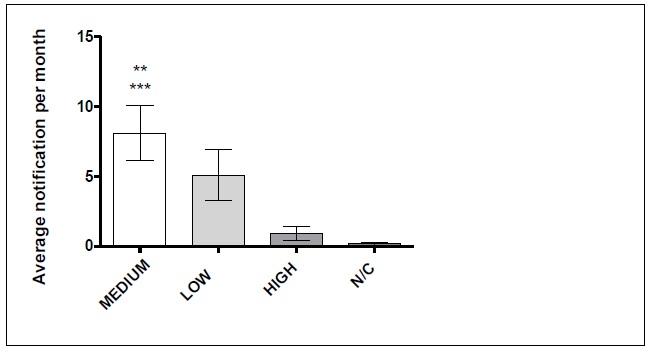
The data was expressed as percentage, average ± SEA, p<0,05
(*)Differs statistically from the Medium group
(**)Differs statistically from the N/C group
(***) N/C: no classification
FIGURE 1
: Risk classification according to NOTIVISA. Sentinel Hospital in São
Luís-Maranhão, January-December, 2015
Regarding adverse event reports, it was observed that the products of Medium Risk and High Risk also presented 6 (35.29%), making a total of 70.6% of adverse events. In Brazil, most of the products registered are of Medium Risk - Class II23 and are subject to a simplified registration process21, which may be one of the causes of so many technical complaints involving hospital medical articles.
Therefore, it is necessary to increase the control parameters and a possible reclassification of the risk of such products33, with the proper monitoring of the same by the companies and Anvisa together. The holder of the health product registration must structure and implement a techno-surveillance system in order to ensure effective risk management with mandatory notification in any failure associated with their articles 13. On the other hand, the Risk Management, as the object of Anvisa, is responsible for promoting post-marketing surveillance in the hospital, identifying incidents and applying procedures that minimize adverse consequences, in order to obtain quality and safety in the patient care.
CONCLUSION
The medical and hospital articles are diverse and widely used within the health facilities, which ratifies the importance of monitoring these through risk management, which has in its scope to provide an efficient control of the quality of medical hospital articles. However, for effective risk management, it is necessary the collaboration of all professionals, who through educational strategies must be improved with the final intention to modify the existing culture and to contribute to the quality of the care provided. It is also presumed a system of active pre-qualification of health products, that provides an appropriate selection that demands quality of these products before being acquired by the health establishment. The existence of a risk minimization plan in the area of Techno-surveillance can also contribute to systematize procedures, minimizing the waste and providing the planning of necessary material resources, without affecting the quality of the services offered.
One limitation that has been found in the study was the description of the quality deviation presented in the product, which made it difficult to systematize the data. Many descriptions were not self explanatory or detailed, and this may reflect the superficial understanding of the concept of quality deviation, and therefore, training is required for the professionals in order to adequately fill in the fields of information on health product complaints. This process is essential to seek and understand the causes involved in the occurrence of the event, implementing later barriers that can avoid recurrence of similar events.
REFERENCES
1.Ministério da Saúde (Br). Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Departamento de Ciência e Tecnologia. Política Nacional de Gestão de Tecnologias em Saúde. Brasília (DF): Ministério da Saúde; 2010.
2.Ministério da Sáude (BR). Conselho Nacional de Secretários de Saúde. Ciência e Tecnologia em Saúde, Brasília (DF): Ministerio da Sáude; 2007.
3.Luppi CBH. Gerenciamento do risco sanitário hospitalar da área de tecnovigilância: análise retrospectiva e prospectiva das notificações de queixas técnicas, incidentes e eventos adversos relacionados ao uso de equipamentos, materiais médico-hospitalares e kit de diagnóstico [tese de doutorado]. Botucatu: Universidade Estadual Paulista; 2010.
4.Costa EA, organizadora. Vigilância Sanitária: temas para debate. Fundamentos da vigilância sanitária. Salvador (BA): Editora da Universidade Federal da Bahia. [Scielo-Scientific Electronic Library Online]. 2009 [citado 2016 abr 27]; p. 67-70. Disponivel em: http://books.scielo.org.
5.Rumel D, Petramale A, Trindade E, et al. Projeto Hospitais Sentinela: estratégia brasileira para vigilância sanitária de produtos de saúde na fase pós-comercialização. Revisa. 2006; 2(2): 95-104.
6. Ministério da Sáude (BR). Agência Nacional de Vigilância Sanitária. Sistema de qualidade de tecnovigilância de produtos para saúde. Brasília (DF): Ministerio da Sáude; 2003.
7.Gil RB. O processo de notificação da queixa técnica de material de consumo de uso hospitalar no contexto do gerenciamento de recursos materiais em um hospital universitário público [dissertação de mestrado]. Ribeirão Preto: Universidade de São Paulo; 2011.
8.Ministério da Saúde (Br). Agência Nacional de Vigilância Sanitária. Rede de Hospitais Sentinela. Brasilia (DF): Ministério da Saúde; 2010.
9.Kuwabara CCT, Évora YDM, Oliveira MMB. Gerenciamento de risco em tecnovigilância: construção e validação de instrumento de avaliação de produto médico-hospitalar. Rev. Latino-Am. Enfermagem [Scielo-Scientific Electronic Library Online]. 2010. [citado em 2016 abr 27]; 18(5). Disponível em: http://www.scielo.br/pdf/rlae/v18n5/es_15.pd.
10.Ministerio de Sanidad y Política Social. Estudio IBEAS: Prevalencia de efectos adversos en hospitales de Latinoamérica. Madrid: Ministerio de Sanidad y Política Social; 2010.
11.Ministério da Saúde (Br). Agência Nacional de Vigilância Sanitária. Assistencia segura: uma reflexão teórica aplicada à pratica. Brasilia (DF): Ministério da Saúde; 2013.
12. Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Resolução RDC n.º 2, de 25 de janeiro de 2010, dispõe sobre o gerenciamento de tecnologias em saúde em estabelecimentos de saúde. Brasília(DF): Ministério da Saúde; 2010.
13.Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Resolução RDC n.º 67, de 21 de dezembro de 2009, dispõe sobre boas práticas de manipulação de preparações magistrais e oficinais para uso humano em farmácias. Brasília (DF): Ministério da Saúde; 2009.
14.Monzani AA, Santana ARCMBF, Miasso AI, Cassiani SHB. A dificuldade dos enfermeiros frente aos relatos de incidentes. Nursing. 2006; 99(8): 958-60.
15.Wang SV, Schneeweiss S, Gagne JJ, Maclure M. "First-Wave" bias when conducting active safety monitoring of newly marketed medications with outcome-indexed self-controlled designs. Am. J. Epidemiol. 2014; 180(6): 636-644
16.Coloma PM, Schuemie MJ, Trifirò G, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011; 20(1):1–11.
17.Platt R, Carnahan RM, Brown JS, et al. The U.S. Food and Drug Administration's mini-sentinel program: status and direction. Pharmacoepidemiol Drug Saf. 2012; 21(suppl 1):1–8.
18.Stang PE, Ryan PB, Racoosin JA, et al. Advancing the science for active surveillance: rationale and design for the observational medical outcomes partnership. Ann Intern Med. 2010; 153(9):600–606.
19.Ministério da Saúde (Br). Agência Nacional de Vigilância Sanitária. Relatório anual de atividades da anvisa 2006. Brasília (DF): Ministério da Saúde, 2007.
20.Azulino ACO, Costa MHA, Carvalho MN, Moreira AS, Oliveira AF, Pinto ACG. Queixas técnicas realizadas pelos profissionais da saúde, relacionadas aos produtos utilizados em hospital sentinela de Belém – Pará. Rev. Bras. Farm. Hosp. Serv. Saúde. 2013; 4(3):13-16.
21.Agência Brasileira de Desenvolvimento Industrial. Manual de registro e cadastramento de materiais de uso em saúde. Brasília (DF): Agência Brasileira de Desenvolvimento Industrial, 2011.
22.Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Resolução RDC n.º 56, de 06 de abril de 2001. Estabelece os requisitos essenciais de segurança e eficácia aplicáveis aos produtos para saúde. Brasília(DF): Ministério da Saúde; 2001.
23.Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Boletim Informativo de Tecnovigilância - BIT. Comportamento dos materiais médico-hospitalares no Brasil a partir dos dados de notificação em Tecnovigilância. Brasília(DF): Ministério da Saúde; 2012.
24.Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Resolução RDC nº 185, de 22 de outubro de 2001, dispõe do registro, alteração, revalidação e cancelamento do registro de produtos médicos na Agência Nacional de Vigilância Sanitária. Brasília(DF): Ministério da Saúde; 2001.
25.Calandrine EF, Silva JYT, Lima ESF, Viana LFO, Calandrine CO. Atuação da tecnovigilância em um hospital sentinela no município de Belém–Pará. In: Anais do 17° Seminário Nacional de Pesquisa em Enfermagem; 2013 jun 03-05; Natal, Brasil. Rio Grande do Norte (RN): Associação Brasileira de Enfermagem; 2013. p. 2320 – 2322.
26. Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Cartilha de Notificações em Tecnovigilãncia. Brasília(DF): Ministério da Saúde; 2003.
27.Braithwaite J, Westbrook M, Travaglia J. Attitudes toward the large-scale implementation of an incident reporting system. Intern J Qual Health Care. 2008; 20(3):184–191.
28.Capucho HC, Arnas ER, Cassiani SHB. Segurança do paciente: comparação entre notificações voluntárias manuscritas e informatizadas sobre incidentes em Saúde. Rev Gaucha Enferm. 2013; 34(1):164-72.
29.Runciman WB, Williamson JA, Deakin A, et al. An integrated framework for safety, quality and risk management: an information and incident management system based on a universal patient safety classification. Qual Saf Health Care. 2006; 15(suppl 1):82-90.
30.Lima PF, Cavassini ACM, Silva FAT, Kron MR, Gonçalves SF, Spadotto A, et al. Queixas técnicas e eventos adversos a medicamentos notificados em um hospital sentinela do interior de São Paulo, 2009-2010 Epidemiol. Serv. Saúde. 2013; 22(4):679-686.
31.Moreira IA, Bezerra ALQ, Paranaguá TTB, Silva AEBC, Azevedo Filho FM. Conhecimento dos profissionais de saúde sobre eventos adversos em unidade de terapia intensiva Rev enferm UERJ, Rio de Janeiro. 2015; 23(4):461-7.
32.Bezerra ALQ; Camargo e Silva AEB; Branquinho NCSS; Paranaguá TTB. Análise de queixas técnicas e eventos adversos notificados em um hospital sentinela. Rev. Enferm. UERJ, Rio de Janeiro, 2009; 17(4):467-72.
33.Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Boletim Informativo de Tecnovigilância - BIT. Estudo de caso: Tubo Endotraqueal. Brasília(DF): Ministério da Saúde; 2012.
34.Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Nota técnica n° 01/2015: Orientações gerais para a notificação de eventos adversos relacionados à assistência à saúde. Brasília(DF): Ministério da Saúde; 2015.
36.Ministério do Desenvolvimento. Indústria e Comércio Exterior Instituto Nacional de Metrologia, Qualidade e Tecnologia. Portaria n.º 332, de 26 de junho de 2012 dispões da aprovação do regulamento de avaliação da conformidade para luvas cirúrgicas e de procedimento não cirúrgico de borracha natural, borracha sintética e de misturas de borrachas sintéticas. Brasília (DF): Ministério do Desenvolvimento; 2012.
37.Ministério da Saúde (BR). Agência Nacional de Vigilância Sanitária. Pré-qualificação de artigos médico-hospitalares: estratégia de vigilância sanitária de prevenção. Brasília(DF): Ministério da Saúde; 2010.
38.International Standards Organization (ISO) 14971. Medical devices: Application of risk management to medical devices. Genebra: 2000.