
RESEARCH ARTICLES
Reported cases of adverse events following immunization: contribution to nursing care
Nathalya Macedo Nascimento CostaI; Ana Maria Machado LeãoII
I
Nurse graduated from the Nursing School at the University of Rio de Janeiro State, Brazil. E-mail: nathmacedo2010@hotmail.com
II
Nurse, Master, Assistant Professor of the Department of Public Health Nursing of the Nursing School at the University of Rio de Janeiro State. Brazil.
E-mail: ammleao@gmail.com
III
This research awarded the Rachel Raddock Lobo Prize, 2nd place in the 7th National Symposium - The Health Care and Nursing. University of Rio de Janeiro
State, 2014.
DOI: http://dx.doi.org/10.12957/reuerj.2015.14850
ABSTRACT
This quantitative, documentary study aimed to characterize the population affected by post-vaccination adverse events, by sex, age, and the vaccines, and to analyze the events. Data on 329 post-vaccination adverse events was collected in 2014 from 214 notification records completed in 2010-2013 at two municipal health centers in the Rio de Janeiro municipal area. The results show that females and under one year-olds were the most affected. The vaccines that produced most adverse events comprised the adjuvant aluminum hydroxide, with the Tetravalent formulation returning the highest percentage. The most frequent adverse events were mild to moderate. It was concluded that nurses responsible for immunization should have a knowledge of immunobiologics and related adverse events in order to prevent them.
Keywords: Vaccination; epidemiological surveillance; notification; nursing.
INTRODUCTION
With the development and discovery of the first vaccines in an attempt to control infectious diseases, immunization was a mandatory practice, generating disgust and conflicts in the population as the Vaccine Revolt. One cannot confirm whether the vaccines were totally risk-free, but it required courage from professionals to develop vaccine activities. During the 1980s and 1990s, there was a decrease in the incidence of immunopreventable diseases due to the clarification of the population and health professionals about the safety of vaccines; however, with the growing number of doses applied, the fear of post-vaccination adverse events arises 1,2.
The vaccine is an immunobiological product consisting of one or more immunizing agents in various forms: live attenuated bacteria, killed or avirulent bacteria, bacterial components, toxins obtained from cultures of bacteria, live attenuated viruses, inactivated viruses and virus fraction. Moreover, immunizing agents also have other components, such as: suspension liquid composed by distilled water and physiological saline solution; preservatives; stabilizers and antibiotics to prevent the growth of contaminants and adjuvants increasing the immunogenic power of the vaccine3.
The post-vaccination adverse events can be understood as any serious, undesirable or unexpected signs or symptoms manifested in an individual who has received some vaccine. They can be classified according to the cause: induced by the vaccine due to its component; errors related to the preparation, handling or administration technique; and coincident, that is, the event has already existed at the time of vaccination, but it only manifested after application of the product. Thus, depending on the intensity and manifestations occurred, suspected cases of post-vaccination adverse events should be investigated and reported2-4.
In this context the research problem arises: What are the characteristics of post-vaccination adverse events from 2010 to 2013, reported in local health centers?
There is high frequency of mild local and systemic adverse events, with epidemiological surveillance targeted for moderate and severe events2-4.
The reason for the interest in this theme emerged from the first author of this paper's experience as intern for 24 months in the Extension Project Vaccinating the Community, from the Nursing School of the Rio de Janeiro State (ENF/UERJ), under the guidance of Professor Ana Maria Machado Leão, in the Undergraduate Program in Nursing. During this period, it was observed that surveying the vaccination history of the people during the screening, the right technique in the preparation and application of vaccines and guidelines after vaccination are of paramount importance to avoid adverse events.
From this perspective, the study becomes relevant, by the knowledge of registered post-vaccination adverse events, and of the characteristics of the affected population in order to ensure safe performance of nurses and the prevention of events.
Given these considerations, it was proposed as research objectives - to characterize the population affected by the post-vaccination adverse events, according to sex and age, to identify vaccines and to analyze the post-vaccination adverse events.
LITERATURE REVIEW
The Programa Nacional de Imunização (National Immunization Program - PNI) contributes to the control of immunopreventable diseases, by administering immunizations at health units and through campaigns. In order to maintain the high vaccination coverage and safety of vaccines, the PNI implemented in 1991 the Sistema de Vigilância Epidemiológica de Eventos Adversos pós–vacinação (Epidemiological Surveillance System for Post-vaccination Adverse Events - SVEAPV), which was one of the most successful systems, and aims to notify, investigate, monitor and standardize appropriate conducts before the events 1.
With the emergence of SVEAPV, health professionals working in health care units had to be trained about the diagnosis and conducts, allowing a greater understanding of the post-vaccination events5.
After the outbreak of the adverse event, the health worker should take notes of data of the person who received the vaccine and its manifestations, in the specific notification form. After its completion, the person should be referred to the local or municipal Epidemiological Surveillance, in the period up to 48 hours, considering thus that the investigation was initiated on time. When events are serious, they shall be notified to the superior hierarchical level immediately, in order to alert the surveillance and obtain guidance on the investigation, if necessary3,4,6,7.
The nurse responsible for the immunization room should have knowledge of vaccines, as well as of the events that they can cause, coordinating the nursing staff under his/her supervision, for immunization activities. Study warns about the paucity of professional knowledge on this theme, reflecting on the decision making and the gaps in the investigation of post-vaccination adverse events that characterize risk to the customer. It is observed that nurses limit themselves to the records of these events only at the local level, reporting them to the epidemiological surveillance2.
METHODOLOGY
This is a descriptive, documentary study with a quantitative approach carried out in two municipal health units, located in the municipality of Rio de Janeiro. These units provide primary care services, covering health promotion, health protection, disease prevention, diagnosis, treatment, rehabilitation and health maintenance. Also, they have the epidemiology sector, responsible for investigating all suspect cases, reporting them to the municipal level. The two units are easily accessible and serve as internship field for undergraduate students from NFE/UERJ.
After approval by the Research Ethics Committee of UERJ, opinion number 642374, authors proceeded to the data collection using the notification forms of post-vaccination adverse events, for they must record the patient information on their adverse reactions to the vaccine. The adverse events selected occurred from 2010 to 2013, according to the records of 216 notification forms, which represented the sample. However, two forms had incomplete filling in the blank of age and were excluded. Thus, the sample was reduced to 214 forms.
The study was conducted in accordance with Resolution No. 466/2012 of the National Health Council8.
Data collection was held in May 2014 and a semi-structured form with four questions was used, of which one was open and three were closed.
To compose the study the following variables were selected: sex, age, vaccines and post-vaccination adverse events.
Data from 2011 to 2013 were collected in the epidemiology sector of the Health Center Maria Augusta Estrella (CMS-MAE), obtaining 149 forms. It is noteworthy that it was not possible to locate the forms of 2010 in this unit; the forms were collected and sent to the Health Department. In the Municipal Health Center Milton Fontes Magarão (CMS-MFM) the 65 printed forms were not filed; there were organizational change in notifications, which are now performed online. Therefore, the data of 2010 of the CMS-MAE and of the whole selected period of CMS-MFM were collected in the Municipal Health and Civil Defense Department of Rio de Janeiro State. We used the Epi-Info program and the notification forms for adverse events.
The following exclusion criteria were selected: notification forms of events occurred outside the selected period, forms filled with illegible handwriting and with incomplete data.
The analysis used the Microsoft Excel Offce 2007 program for tabulation and organization of the findings, allowing synthesizing them into tables, according to descriptive statistics. The discussion of the findings was performed based on literature.
RESULTS AND DISCUSSION
In the selected sample, females predominated, with 130 (61%), since the male had 84 (39%) subjects. There is low demand for health services by men, possibly due to sociocultural factors; while women are the most frequent users of the service9. But there is no direct relationship with the findings of this work, as the age group with more events focused on children under one year old. In the total of analyzed forms, there were 329 adverse events classified as mild, moderate and severe, evidencing more than an event in certain forms. Adverse events are classified according to intensity: severe occurs when there is hospitalization for at least 24 hours, resulting in risk of death; moderate, when there is need for evaluation, laboratory tests and medical treatment; light, when no medical treatment and no additional tests are required, the latter being characterized as nursing practice 1,2,4,5.
The distribution of notifications by age group in the two units showed higher incidence of post-vaccination adverse events in the age range under one year, 136 (64%); whereas the age range from 5 to 12 years old had reached 7 (3%), the lowest incidence, according to Table 1. The most vulnerable are children under one year old, because they have immature immune system and due to the amount of vaccines administered in various doses in this age group, as recommended by the PNI1,6.
TABLE 1
: Distribution of notifications of adverse events by age group of the Municipal Health Centers Milton Fontes Magarão and Maria Augusta Estrella. Rio de
Janeiro, 2010-2013. (N =214)

There was administration of 214 vaccines, which resulted in adverse events, surveyed in the notification forms, as provided in Table 2.
TABLE 2
: Distribution of the administered vaccines that caused adverse events reported by the Municipal Health Centers Milton Fontes Magarão and Maria Augusta
Estrella. Rio de Janeiro, 2010-2013. (N =214)
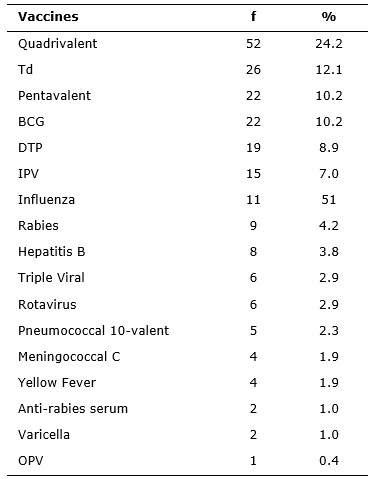
The quadrivalent vaccine, against diphtheria, tetanus and pertussis (whooping cough), combined (DPT) with Haemophilus influenzae type b, showed high incidence of adverse events compared to other vaccines administered. Such reactions are explained by the adjuvant aluminum hydroxide, component found in several vaccines, such as Meningococcal C, Double adult vaccine against tetanus and diphtheria (Td), pentavalent (DTP + Hepatitis B and meningitis caused by Haemophilus influenzae type b), Triple bacterial vaccine, against diphtheria, tetanus and pertussis (DTP) and Hepatitis B; these also have thimerosal as a preservative, except for Meningococcal C vaccine.
Individuals with skin allergy to thimerosal may have local inflammatory process. In this situation, the vaccine is not contraindicated7. The 10-valent pneumococcal vaccine is composed of 10 pneumococcal serotypes conjugated to protein D from Haemophilus influenzae and carried of diphtheria and tetanus toxoid and composed of aluminum phosphate and sodium chloride10.
Vaccines with aluminum hydroxide can cause local inflammation events, stimulating the immune system, and subsequent doses can increase the number of events. To avoid local reactions it is very important to carry out rotational movements of the vaccine vial for homogenization of the solution before aspirating each dose; the administered liquid should be introduced slowly; and positioning should be safe and comfortable, especially in a child to be vaccinated11.
The quadrivalent vaccine is recommended for children from two months of age with three-dose schedule at 2, 4 and 6 months of age, with an extra dose at 15 months of age with the DTP vaccine. Besides aluminum hydroxide as adjuvant, it also contains the pertussis component which may cause adverse events. Vaccines with that component present adverse events with little gravity, usually within 48 hours5,11,12.
BGC had 22 (10.2%) notifications; it was the fourth vaccine that most caused adverse events, as well as the pentavalent. Generally adverse events of BCG are mainly related to technical errors, dose above the suitable volume, deep application, subcutaneously, contamination at the time of preparation, inadequate preservation of the product. This vaccine is a live attenuated bacterium suspension against tuberculosis, which causes events after vaccination, as the evolution of a local node, which progresses to pustule, then crust and ulcers, lasting from 6 to 10 weeks, resulting in a scar. Such events should be clarified those responsible for the child so that they can be attentive to unexpected events that may occur7,13.
It is necessary that the nurse has knowledge and skill as to the intradermal application of this vaccine; enabling this ability to be acquired by other professionals, through training, to prevent errors and techniques and to ensure to the vaccinated person care free of damage13.
The injectable inactivated polio vaccine (IPV) showed only 15 (7%) adverse events. This data corroborates another study that emphasizes the occurrence of only local reactions14.
Hypersensitivity reactions are allergic and may be related to the composition of vaccines grown in embryonated chicken eggs, but those vaccines offer lasting immunity with a single dose, due to the live virus in its composition; some have the potential to cause more serious adverse events such as the triple viral vaccine, yellow fever, influenza and rabies. The last two mentioned are inactivated vaccines that contain killed virus and require more than one dose, which may cause adverse events related to the hyper-immunity with exaggerated doses7. This study points hypersensitivity reaction after two hours of administration of rabies vaccine and generalized urticaria and after application of the vaccine against yellow fever, whereas Viral Triple vaccine and Influenza did not expressed anaphylactic reaction to chicken egg.
Oral Polio Vaccine (OPV) revealed 1 (0.3%) notification and the vaccine against Varicella, 2 (0.6%). Both vaccines are composed of live attenuated viruses and anti-rabies serum is composed of of immunoglobulins, such as phenol, thimerosal or tricresol (preservatives) and sodium chloride (solvent); their adverse events occur from 6 to 12 days7. These three vaccines caused few events in this research, featuring local events such as myalgia after the varicella vaccine, rash, pruritus, hypersensitivity after the anti-rabies serum and fever in the case of OPV.
Oral vaccine against Rotavirus is a vaccine made with attenuated viruses isolated from humans; adverse events recorded were resulting from the application, simultaneous or not, of other vaccines7. Events of stools with streaks of blood and cyanosis have been reported. Other studies highlight the serious adverse events of this vaccine, such as intestinal obstruction; due to ischemic process, there may be blood in the stool, in the most severe cases. It is highlighted the importance of applying the vaccine in children up to 24 months, which prevents intussusception; if applied outside the recommended period, the risk of the event increases 5,15.
There was a high frequency of vaccines composed of aluminum hydroxide that caused adverse effects such as local reactions. In such cases, more than one dose is recommended. Another significant percentage refers to the live virus vaccines such as Triple Viral Vaccine and Yellow Fever.
It is important to be aware about the contraindications for the administration of vaccines, as in the cases of patients with congenital or acquired immunodeficiency, use of corticosteroids with immunosuppressive scheme, suffering from malignant neoplasia, use of chemotherapy and radiation. There are also false contraindications, as mild or moderate diarrhea, allergic respiratory diseases, skin disease, malnutrition and hospitalization, that cause impairment in vaccination coverage due to lack of information by the vaccinated people. The administration of live virus vaccines should be delayed in cases of individuals with severe febrile disease, bone marrow transplantation, use of immunoglobulins, and treatment with low doses of corticosteroids 3,7,16.
Of the 329 adverse events, local reactions were the most significant, such as pain, redness, heat, induration, edema and erythema, which were frequent in almost all vaccines except for oral vaccines, such as OPV and rotavirus; BCG did not present events such as pain, redness and heat. Rash was revealed by rabies, Pneumococcal, Triple Viral, DTP and quadrivalent vaccines. In the triple viral vaccine, the rash is associated with components against measles and rubella7. The approach adopted for local reactions is to maintain the immunization schedule6. See Table 3.
TABLE 3
: Distribution of adverse events reported of Municipal Health Centers Milton Fontes Magarão and Maria Augusta Estrella. Rio de Janeiro, 2010-2013. (N=329)
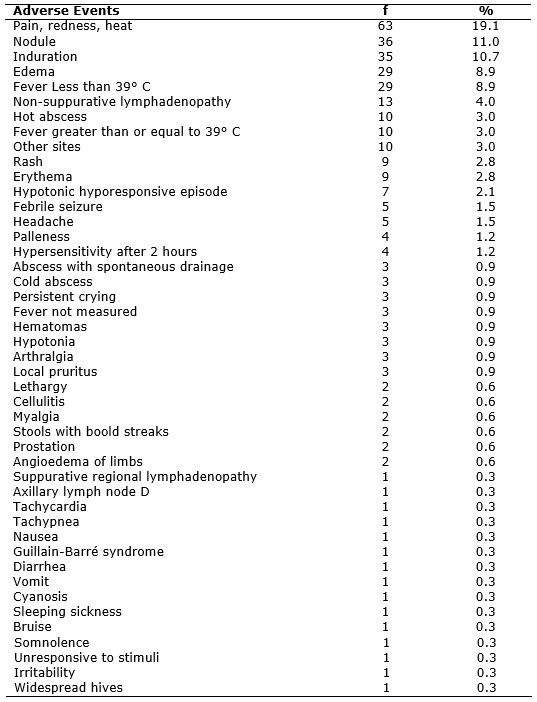
Local irritation and muscle damage are caused by incorrect insertion of the needle. It is recommended to use long needles to reach the muscle tissue, for when the administration takes place in the subcutaneous tissue, it can cause irritation, inflammation or local necrosis7,11,12.
The nodule may appear at the injection site, being absorbed slowly over weeks7. Vaccines that showed nodules as adverse events were quadrivalent, BCG, Meningococcal C, Pneumococcal 10-valent, Hepatitis B, and Pentavalent.
The administration of antigens causes a physiological response, increasing axillary temperature, higher or lower than 39° C; other events are local abscesses due to inflammation2,7. Vaccines that caused fever in this study were the quadrivalent, Pentavalent DTP, Td, Hepatitis b, Rabies, Triple Viral vaccine, OPV, Meningococcal C, Pneumococcal 10-valent, Influenza and Rotavirus. Hot abscess was recorded in 10 (3%) notifications, it was caused by vaccines: BCG, Td, quadrivalent and Pentavalent. Such events suggest secondary infection2,7. Cold abscess had 3 (0.9%) notifications and took place after the Pentavalent and the DTP; it was explained by inadequate inoculation, subcutaneously, when it should have been intramuscular, featuring a technical error7. Based on the findings, it is noticed that the two events feature technical errors made by professionals, highlighting the secondary infection as the most frequent.
BCG vaccine showed abscess with spontaneous drainage, according to 3 (0.9%) notifications, indicating localized inflammatory signals and 1 (0.3%) with suppurative regional lymphadenopathy. These findings indicate incorrect technique at the time of application3. BCG, influenza, Td, and Triple Viral vaccine showed non-suppurative regional lymphadenopathy - 13 (4%). This event should be monitored up to its disappearance, usually within 4 months 1. The lymphadenopathy resulting from the application of the Triple Viral vaccine is associated with the rubella component7.
The conduct in face of adverse events determines treatment with analgesics in the case of pain; cold compresses the first 24-48 hours after application, and in the case of abscesses, nurses should direct the client to medical evaluation7.
The qualification of nurses in the vaccination room is of great importance as it must follow the rules established by PNI, possessing adequate knowledge of post-vaccination adverse events and prevention of them, which must be passed on to other professionals, through training activities4.
There were some severe events in this study, such as the hypotonic hyporesponsive episode (HHE), whose clinical feature arises in the first 48 hours after administration of the vaccine and can last for minutes or days, followed by pallor, disappearance of muscle tone and absence of response to stimuli. Most children present irritability and fever6. Vaccines that caused HHE were Quadrivalent, DTP and the Pneumococcal 10-valent. Study shows the DTP and Quadrivalent associated with HHE because of their pertussis component; it also occurs in other cases such as pneumococcal vaccines7.
Febrile seizure was recorded in 5 (1.5%) notifications; it is manifested in the first 72 hours after vaccination. Persistent crying appears, usually within 24 hours, with no pathophysiological explanation. Vaccines that have provided these events were Quadrivalent and Pentavalent. Fever higher than 39º C indicates risk of seizures7. Administration of high concentrations of tetanus toxoid may cause irritability7. Irritability due to the Td vaccine was mentioned in 1 (0.3%) notification. Such an event is related to the high number of doses or technical error.
Hypersensitivity reactions were caused by Quadrivalent, anti-rabies serum and Pneumococcal 10-valent vaccines. According to the Ministry of Health, it has not yet been possible to relate hypersensitivity to any component of Quadrivalent vaccine7. The anti-rabies serum may cause serum sickness, which is a hypersensitivity reaction, accompanied by fever, skin, joint and linfoganglionar manifestations, which occurs due to the deposition of immune complexes in blood vessels10. There was also myalgia after the yellow fever vaccine. In another study, myalgia was not mentioned as an event due to vaccine7. Hypersensitivity occurred also in Influenza; this event is more frequent in individuals who have never received previous dose 17.
Meningococcal C conjugated caused, as adverse events, diarrhea and vomiting; in the official protocols there is no justification for the occurrence of those events. In the reports raised, the anti-rabies serum presented itching. This finding corroborates a study that described such an event as one of the cutaneous manifestations7.
Some findings in this research are not described in the Ministry of Health protocols, such as tachycardia and tachypnea, caused by the DPT vaccine; the lack of response to stimulation after administration of Hepatitis b vaccine; angioedema of limbs due to the Pentavalent vaccine; bruise after Rabies vaccine; lethargy after Hepatitis b vaccine and DTP; prostration due to Td and Hepatitis B; cellulite after DTP and Td; arthralgia, characterized by joint pain in DTP; pruritus in Influenza and Td; pallor after the BCG, Rotavirus and DTP vaccines; hematoma in the IPV, Rabies and Pentavalent vaccines; and headache after Pneumococcal vaccine and Td.
Another serious event with not very significant percentage was the Guillain-Barré Syndrome (GBS), with only 1 (0.3%) notification, caused by Influenza vaccine. This finding features peripheral nervous system, leading to various degrees of motor weakness, reaching the upper limbs and the face. This syndrome is associated with Influenza vaccine, since the first appearance in 1976. It is noteworthy that the very Influenza virus can trigger GBS, but this is not very frequent18.
CONCLUSION
Although vaccines can cause post-vaccination adverse events, due to its components, technical errors or characteristics of the vaccinated person him/herself, it is noteworthy that vaccination is of great importance for the prevention of infectious diseases, meaning benefit for the population.
Females had a higher incidence of adverse events, the age range under one year old was the most affected, due to immunological immaturity and the coincidence with the period that they receive greater amount of vaccines, according to the PNI.
Quadrivalent vaccine was the one that most produced adverse events due to the fact that it is constituted by aluminum hydroxide adjuvant, which is also found in several vaccines; there is highlight for local reactions.
Regarding the BCG vaccine, technical errors were the most stressed. This research presented a small sample, referring to two health units, which meant a limitation of the study and requires reapplication. It is hoped that this study may collaborate on other scientific works and to encourage nursing care from the reflections on changes in care because the nurse plays a key role in the National Immunization Program and in monitoring post-vaccine reactions.
REFERENCES
1.Araújo TME, Carvalho PMG, Vieira RDF. Análise dos eventos adversos pós-vacinais ocorridos em Teresina. Rev Bras Enferm. 2007; 60:444-8.
2.Bissetto LHL, Cubas MR, Malucelli A. A prática de enferagm frente aos eventos adversos pós- vacinação. Rev esc enferm USP. 2011; 45:1128-34
3.Ministério da Saúde (Br). Manual de normas de vacinação. Brasília (DF): Secretaria de Comunicação; 2001.
4.Alves H, Domingos LMG. Manejo de eventos adversos pós vacinação pela equipe de enfermagem: desafio para o cuidado. Rev enferm UERJ. 2013; 21: 502-7.
5.Martins RM, Maia, MLS. Eventos adversos pós- vacinais e resposta social. Hist ciêc saúde. 2003; 10: 807-25.
6.Piacentini S, Moreno LC. Eventos adversos pós vacinais no município de Campo Grande (MS, Brasil). Ciênc saúde coletiva. 2011; 16: 531-6.
7.Ministério da Saúde (Br). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de vigilância epidemiológica de eventos adversos pós – vacinação. Brasília (DF): Editora MS; 2008.
8.Ministério da Saúde (Br). Conselho Nacional de Saúde. Que define as Normas reguladoras de pesquisa envolvendo seres humanos: Resolução nº 466/2012. Brasília (DF): Fundação Oswaldo Cruz; 2013.
9.Campos EC, Sudan LCP, Mattos EDM, Fidelis R. Fatores relacionados à vacinação contra a gripe em idosos: estudo transversal, Cambé, Paraná, Brasil. Cad Saúde Publica. 2012; 28: 878–88.
10.Ministério da Saúde (Br). Proposta para Introdução da Vacina Pneumocócica 10 - Valente (conjugada) no calendário básico de vacina da criança. Brasília (DF):Editora MS; 2010.
11.Jesus DM, Bastos MA, Carvalho EC. Estudo dos eventos adversos provocados pela vacina tetravalente. Rev enferm UERJ. 2004; 12: 299 -305.
12.Freitas FRM, Saton HK, Aranda BAF, Pacheco MP, Waldman EA. Eventos Adversos pós vacina contra a difteria, coqueluche e tétano e fatores associados à sua gravidade. Rev Saude Publica. 2007; 41: 1032-41.
13.Fassarella CS, Santos CV, Rosa LS. A resposta do profissional de enfermagem na aplicação da vacina BCG sob óptica da segurança do paciente. Rev Rede de Cuidados em Saude. 2013; 7: 1-10 .
14.Ministério da Saúde (Br). Informe Técnico da Vacina Inativada Poliomielite (VIP). Brasília (DF): Editora MS; 2012.
15.Secretaria do Estado de São Paulo. Vacina contra o rotavírus. Rev Saude Publica. 2006; 40: 355-8.
16.Carvalho ALA, Oliveira DLA, Pereira W, Souza FGM. Hospitalização como oportunidade para atualizar o calendário básico de vacinação: uma experiência realizada no hospital universitário em São Luis- MA. Rev RENE. 2004; 5:89-94.
17.Ministério da Saúde (Br) Informe Técnico Campanha Nacional de Vacinação contra a Influenza. Brasília (DF): Editora MS; 2014.
18.Ferrarini MAG, Scattolin MAA, Rodrigues MM, Resende MHF, Santos ICLS, Iazzetti AV. Sindrome de Guillain – Barré em associação temporal com a vacina Influenza A. Rev Paul Pedritr. 2011; 29:685-8.