RESEARCH ARTICLES
Hepatitis B vaccination in chronic renal failure patients on hemodialysis
Letícia Pimenta LopesI; Sheila Araujo TelesII; Elen Almeida RomãoIII; Silmara Elaine Malaguti ToffanoIV; Deborah Ferreira Noronha de Castro RochaV; Elucir GirVI
IRegistered Nurse, PhD Student, Ribeirão Preto College of Nursing at the University of São Paulo. Ribeirão Preto, São Paulo, Brazil. Email: letylopes@yahoo.com.br.
ABSTRACT: Individuals on hemodialysis are at high risk of infection by Hepatitis B virus and are, therefore, a target population for vaccination against Hepatitis B. This retrospective cohort study aimed to evaluate Hepatitis B vaccination monitoring of patients who started hemodialysis in 2005 and continued in follow-up for up to four years in Ribeirão Preto, São Paulo State. Of the population of 102 individuals, only 39.2% were on record as previously vaccinated against Hepatitis B, while 35.3% received the full vaccination schedule. The majority received a three-dose regimen (40 mcg), and 72.2% developed protective titers of anti-HBs. Of the 62 individuals with no record of previous vaccination, 22.6% remained on hemodialysis for more than 42 months. Findings highlight the urgent need for more effort by policy managers and health professionals in surveillance of Hepatitis B vaccination at hemodialysis centers in the region.
Keywords: Renal dialysis; chronic renal insufficiency; Hepatitis B vaccines; Hepatitis B antibodies.
INTRODUCTION
The Hepatitis B Virus (HBV) has been a cause of acute and chronic hepatitis, cirrhosis and hepatocellular carcinoma. Approximately two billion people have been infected with HBV worldwide, and around 350 million are chronic carriers of this virus. It is also estimated that 600,000 people die each year due to the acute or chronic consequences of hepatitis B1.
HBV is a hepatotropic virus that belongs to the Hepadnaviridae family, therefore men are its natural reservoir2,3. It is a resistant virus, able to remain infectious for more than a week on surfaces of the environment4. HBV can be found in blood and body fluids, such as wound exudates, semen, cervical and vaginal secretions, as well as in saliva. Therefore, this virus can be transmitted by sexual, parenteral, vertical and horizontal transmission2.
Subjects with chronic renal failure (CRF) are especially susceptible to HBV infection, and the sources of infection include: blood transfusions, contamination of dialysis equipment and cross-contamination through environmental surfaces5. Thus, studies have shown high rates of positivity to the marker of HBV infection in hemodialyzed patients6,7.
Vaccination is a low-cost, safe and effective method to prevent Hepatitis B8. The conventional immunization schedule for healthy adults is composed of three doses of 20 mcg each, at months 0,1 and 6, and the route of administration is the deltoid muscle. The detection of anti-HBs titers ³ 10 mIU / mL, 45 to 60 days after the third dose, indicates protection against Hepatitis B9. Some factors have been associated with vaccine non-response, such as advanced age, obesity, smoking, administration of the vaccine in the dorsogluteal region and immunosuppression10.
CRF patients are often immunosuppressed and they present a lower vaccine response when compared to healthy subjects. Thus, immunization schedules reinforced with four doses (0, 1, 2 and 6 months), using double the standard dose, are recommended for this group. In addition, for these individuals, as opposed to what is recommended for the healthy population, a booster dose is indicated if the title of anti-HBs declines to less than 10 mUI/ml5.
Hence, the purpose of this study was to evaluate the monitoring of vaccination against Hepatitis B in hemodialysis centers from Ribeirao Preto, São Paulo.
LITERATURE REVIEW
Research have shown that patients with chronic renal failure (CRF) respond better to hepatitis B vaccine when this is administered as early as possible, before the need for renal replacement therapy, or immediately after the beginning of the hemodialysis program5.
There is evidence of reduction of the endemicity of hepatitis B in hemodialyzed patients after implementation of vaccination against hepatitis B. In the United States, in 1976, the prevalence of HBV infection was approximately 8%, and this rate dropped to 1% in 200211.
In Brazil, a study conducted in hemodialysis centers in Goiânia, Goiás, showed a decrease of HBV infection of approximately 50% between 1995 (12%) and 1999 (5.8%)12. In 2006, researchers found an overall prevalence of 2.4% for this infection in all hemodialysis centers of Goiás6.
However, this situation is not homogeneous in our country, and variable rates of hepatitis B have been found in hemodialysis centers. In São Paulo, São Paulo, authors reported a prevalence of HBV infection of 15.4% in two hemodialysis centers13, and, in Santa Catarina, a 10% rate14 was found. In Tocantins and Minas Gerais, prevalences of 4.4% and 4% were found15,16. Still, outbreaks of hepatitis B are not uncommon17.
As of the publication of a resolution entitled Portaria 2042, in 1996, vaccination against HBV became mandatory for all CRF patients on hemodialysis in Brazil. In 2004, the Collegiate Directory Resolution (RDC, as per its acronym in Portuguese) no. 154 ratified this regulation. In addition, the National Immunization Program (PIN, as per its acronym in Portuguese) recommends, and offers for free, the vaccine for hemodialyzed patients since the early 90s9. However, there are no studies about the actual implementation of these regulations in hemodialysis centers in Brazil.
METHODOLOGY
This was a retrospective cohort study, developed in four hemodialysis units that assist subjects with CRF in the city of Ribeirão Preto, São Paulo.
In this study, the units were denominated as A, B, C and D, being one public and three private, all linked to the Unified Health System (SUS, as per its acronym in Portuguese). These centers assist dialysis patients from Ribeirão Preto and other cities in the region.
The study population consisted of 102 patients with chronic renal failure undergoing hemodialysis treatment. Inclusion criteria were: being at least 18 years old; being a patient with CRF and having started hemodialysis from January 1st to December 31st of 2005. The medical records of all patients were analyzed up to December 2009, that is, up to four years of follow-up in the dialysis unit.
Data were collected from May 2009 to April 2011. The source of information for this study consisted of review of the medical records of each patient of the hemodialysis units, of which three units (B, C and D) were also used the database of the nephrology clinic management system. The data collected were subsequently transcribed into a form containing sociodemographic questions and information on vaccination against Hepatitis B. This form was validated by two experts in the field regarding its form and content.
The research proposal was approved by the Research Ethics Committee of the University Hospital of the Ribeirão Preto School of Medicine, University of São Paulo. (Process no. 11134/2008).
Data were analyzed using descriptive statistics (absolute frequency, relative frequency and central tendency measures - mean and median) by means of the Statistical Package for Social Sciences (SPSS), version 18.0.
RESULTS AND DISCUSSION
Of the 102 patients studied, 25 (24.5%) were from unit A, 4 (3.9%) were from unit B, 51 (50%) from C, and 22 (21.6%) from D.
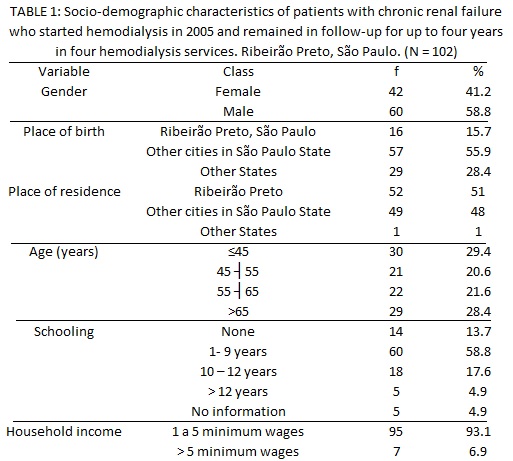
Data regarding sociodemographic characteristics show that 58.8% of subjects were male, and 15.7% and 51% were natural and proceeding from Ribeirão Preto, São Paulo, respectively. Most subjects were 45 years or older and reported having a family income of one to five minimum wages. Almost half (45.1%) presented incomplete elementary school, as presented in Table 1.

Regarding the base diagnosis, 49% of patients had hypertensive nephrosclerosis / arterial hypertension (AH); 34% hypertensive nephrosclerosis/AH and diabetic nephropathy/Diabetes Mellitus; 6% diabetic nephropathy/AH; 4% glomerulonephritis and hypertensive nephrosclerosis/AH; 3% polycystic kidney disease; 3% unknown etiology and 1% lupus nephritis.
Patients on hemodialysis have a high risk of contracting HBV, since sources of infection are various in the unit. In light of this, vaccination against hepatitis B should be performed routinely in hemodialyzed patients, as a prophylactic measure agaimst this infection5.
The lack of monitoring of diseases such as AH and Diabetes Mellitus can accelerate kidney damage, leading to CRF18. Subjects with CRF on hemodialysis often develop a vaccine response against hepatitis B lower than healthy people5. These subjects present a decreased activation of helper T cells, and compromised function of dendritic cells, which play a critical role in immune response as antigen-presenting cells19. Moreover, many of these subjects are older adults and this factor has been associated with lower vaccine response9,10. Therefore, it is recommended to vaccinate CRF individuals as early as possible in course of the disease20, before the beginning of treatment or in the first few months5.
During the study period, only 37 (36.3%) patients remained in follow-up in the hemodialysis units for four years. The median time for follow-up was 38.5 months, that is, 1184 days (minimum: 2, maximum: 1460 days).
Regarding causes that resulted in discontinuation of follow-up, of 65 (100%) subjects, 34 (52.3%) died, 12 (18.5%) underwent renal transplantation, 13 (20%) were transferred to other institutions. The reasons were not described in the medical records for 6 (9.2%) cases.
As regards vaccination against Hepatitis B, of 102 patients, only 40 (39.2%) had vaccination records. Of these, 36 (90%) underwent the complete vaccination schedule and 4 (10%) had incomplete schedule. In 62 (60.8%) records, this information did not exist.
The complete hepatitis B vaccination schedule was carried out before the beginning of renal replacement therapy in only 13.9% of subjects. The number of subjects who received the complete Hepatitis B vaccination schedule was 35.3%. Of these, 72.2% developed protective titers of anti-HBs.
According to the data obtained from the 36 (100%) patients who received the complete vaccination schedule, most remained on hemodialysis for more than 42 months. As for the 4 (100%) subjects who received incomplete vaccination schedule, 2 (50%) remained on hemodialysis treatment for 7-12 months and 2 (50%) for a period exceeding 36 months. With respect to 62 (100%) subjects whose information on the vaccination schedule was not included in medical records, 14 (22.6%) remained on hemodialysis for more than 42 months.
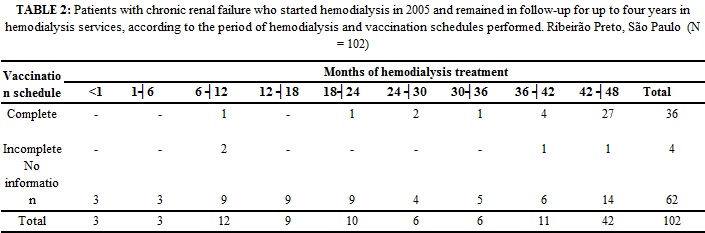
In the present study, only 40 (39.2%) patients had medical records of vaccination against hepatitis B. Furthermore, 14 (22.6%) patients remained on hemodialysis for more than 42 months, without knowledge of the health team about their vaccination status. This finding is alarming and points to the lack of investment in preventing infection of health professionals working in hemodialysis. Indeed, the Hepatitis B vaccine is provided for free to all patients with terminal CRF, and this vaccine is mandatory in Brazil for professionals and patients on hemodialysis. Therefore, one can assume that the failure is systemic, that is, at the local level, health professionals do not comply with these regulations; and in the central level, supervision falls short.
For all subjects with the complete schedule (n = 36), the route of administration of the vaccine used was intramuscular; 34 (94.4%) received the vaccination schedule of three doses of 40 mcg each and 2 (5.6%) patients received four doses of 40 mcg each. There was no information in the medical record about the type of vaccine used in the vaccination schedules.
In CRF subjects, administration of more frequent and more concentrated doses of the vaccine appears to be more effective in inducing protective titers of anti-HBs antibodies5. In the hemodialyzed patients investigated, almost all vaccinated subjects received the three-dose schedule of 40 mcg each at months 0, 1 and 2, and 72.2% developed protective titers of anti-HBs. Other authors reported similar response rates using this schedule21,22.
During the period of this research, the Center for the Epidemiological Monitoring of the State of São Paulo recommended a schedule of three 40 mcg doses23. Currently, the recommendation of the Ministry of Health is followed, that is, four doses of 40 mcg each, at months zero, one, two and six9. Studies have shown that this schedule induces a more robust rate of vaccine response. In fact, studies that used this schedule obtained a vaccine response rate of 89% in hemodialyzed patients in Goiânia, Goiás24, and a seroconversion rate of 93.1% in hemodialyzed patients in Cairo, Egypt25.
A study conducted in China with 156 CRF patients showed a high seroconversion rate to the HBV vaccine, since 70.5% of subjects seroconverted after four 40 mcg doses (0, 1, 2 and 6 months) of vaccine by intramuscular injection26.
Moreover, due to technological advances and constant changes in the hemodialysis environment, research mentions the importance for professionals working on this field to keep up-to-date, so that biosecurity measures are met. In the same study, the adoption of intervention measures aimed at prevention was effective in reducing the incidence of infections in hemodialysis units27.
CONCLUSION
Chronic renal failure patients infected by the HBV may represent true reservoirs of this virus in the dialysis setting, but also in their social environment. Therefore, it is essential vaccination against Hepatitis B is effectively monitored in hemodialysis centers. Public management should conduct effective surveillance of vaccination against hepatitis B in hemodialysis centers. On the other hand, professionals working in hemodialysis units must comply with the operating rules of hemodialysis centers in Brazil, including the prevention and control of hepatitis B.
A limitation of this study is the lack of registration in the medical records of certain variables that would be important to supplement the information collected. It is known that the medical record is a document handled by different professional and which portrays the health care provided to the patient. Therefore, the use of more complete and adequate records is recommended to promote the quality and reliability of information in favor of the control and prevention of this morbidity.
REFERENCES
1. Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:13-21.
2. Hollinger FB, Liang TJ. Hepatitis B Virus. In: Knipe DM, Howley PM. Fields virology, 4ª ed. Philadelphia (USA): Lippincott Williams & Wilkins; 2001. p.2971-3036.
3. International Committee on Taxonomy of Viruses [Internet]. Virus taxonomy. [citado em 11 ago 2013]. Available at: http://www.ictvonline.org/
4. Bond WW, Favero MS, Petersen NJ, Gravelle CR, Ebert JW, Maynard JE. Survival of hepatitis B virus after drying and storage for one week. Lancet. 1981;1:550-1.
5. Centers for Disease Control and Prevention. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1-43.
6. Ferreira RC, Teles SA, Dias MA, Tavares VR, Silva SA, Gomes SA, et al. Hepatitis B Virus infection profile in hemodialysis patients in Central Brazil: prevalence, risk factors, and genotypes. Mem Inst Oswaldo Cruz. 2006;101:689-2.
7. Paniagua R, Villasís-Keever A, Prado-Uribe Mdel C, Ventura-García MD, Alcántara-Ortega G, Ponce de Leon SR, et al. Elevated prevalence of hepatitis B in Mexican hemodialysis patients. A multicentric survey. Arch Med Res. 2010;41:251-4.
8. Romano' L, Paladini S, Van Damme P, Zanetti AR. The worldwide impact of vaccination on the control and protection of viral hepatitis B. Dig Liver Dis. 2011;43:2-7.
9. Ministério da Saúde (Br). Manual dos centros de referência para imunobiológicos especiais. Brasília (DF): Ministério da Saúde; 2013.
10. Assad S, Francis A. Over a decade of experience with a yeast recombinant Hepatitis B vaccine. Vaccine. 1999;18:57-67.
11. Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18:52-61.
12. Teles SA, Martins RM, Gomes SA, Gaspar AM, Araujo NM, Souza KP, et al. Hepatitis B virus transmission in Brazilian hemodialysis units: serological and molecular follow-up. J Med Virol. 2002;68:41-9.
13. Moreira RC, Deguti MM, Lemos MF, Saraceni CP, Oba IT, Spina AM, et al. HBV markers in haemodialysis brazilian patients: a prospective 12-month follow-up. Mem Inst Oswaldo Cruz. 2010;105:107-8.
14. Carrilho FJ, Moraes CR, Pinho JR, Mello IM, Bertolini DA, Lemos MF, et al. Hepatitis B virus infection in haemodialysis centres from Santa Catarina State, Southern Brazil. Predictive risk factors for infection and molecular epidemiology. BMC Public Health. 2004;4-13.
15. Busek SU, Baba EH, Tavares Filho HA, Pimenta L, Salomão A, Correa-Oliveira R, et al. Hepatitis C and hepatitis B virus infection in different hemodialysis units in Belo Horizonte, Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2002;97:775-8.
16. Souza KP, Luz JA, Teles SA, Carneiro MA, Oliveira LA, Gomes AS, et al. Hepatitis B and C in the hemodialysis unit of Tocantins, Brazil: serological and molecular profiles. Mem Inst Oswaldo Cruz. 2003;98:599-603.
17. Lewis-Ximenez LL, Oliveira JM, Mercadante LA, De Castro L, Santa Catharina W, Stuver S, et al. Serological and vaccination profile of hemodialysis patients during an outbreak of hepatitis B virus infection. Nephron. 2001;87:19-26.
18. Frazão CMFQ, Ramos VP, Lira ALBC. Qualidade de vida de pacientes submetidos a hemodiálise. Rev enferm UERJ. 2011; 19:577-82.
19. Lim WH, Kireta S, Russ GR, Coates PT. Uremia impairs blood dendritic cell function in hemodialysis patients. Kidney Int. 2007;71:1122-31.
20. Labriola L, Jadoul M. The decades-long fight against HBV transmission to dialysis patients: slow but definite progress. Nephrol Dial Transplant. 2010;25:2047-9.
21. Bock M, Barros E, Veronese FJ. Hepatitis B vaccination in haemodialysis patients: a randomized clinical trial. Nephrology. 2009;14:267-72.
22. Fernandez E, Betriu MA, Gómez R, Montoliu J. Response to the hepatitis B virus vaccine in haemodialysis patients: influence of malnutrition and its importance as a risk factor for morbidity and mortality. Nephrol Dial Transplant. 1996;11:1559-63.
23. São Paulo (Estado). Secretaria de Estado da saúde de São Paulo. Centro de vigilância epidemiológica “Prof. Alexandre Vranjac. Guia de orientações técnicas Hepatite B e C [Internet]. São Paulo; 2002 [citado em 9 fev 2011]. 47 p. Available at: ftp://ftp.cve.saude.sp.gov.br/doc_tec/outros/hepa_guia03.pdf
24. Teles SA, Martins RMB, Lopes CLR, Carneiro MAS, Souza KP, Yoshida CFT. Immunogenicity of a recombinant Hepatitis B vaccine (Euvax-B) in haemodialysis patients and staff. Eur J Epidemiol. 2001;17:145-9.
25. Ibrahim S, El-Din S, Bazzal I. Antibody level after hepatitis-B vaccination in hemodialysis patients: impact of dialysis adequacy, chronic inflammation, local endemicity and nutritional status. J Natl Med Assoc. 2006;98:1953-7.
26. Lin S, Liu J, Wang S, Wang I, Tsai C, Liu Y, et al. Association of response to Hepatitis B vaccination and survival in dialysis patients. BMC Nephrol. 2012;13:97.
27. Lazzarini FAZ, Andrade D, Rossi LA, Ferraz AEP. Incidência de soroconversão para o vírus da hepatite C após a implementação de programa de prevenção e controle em unidade de hemodiálise. Rev Latino-Am Enfermagem. 2000;8:7-12.