RESEARCH ARTICLES
Development of an instrument to identify nurses' practice in radiodermatitis
Marceila de AndradeI; Maria José ClapisII; Claudia Benedita dos SantosIII; Thais de Oliveira GozzoIV
INurse, Master in Health Sciences. Ribeirao Preto School of Nursing. University of Sao Paulo. Ribeirao Preto, Sao Paulo, Brazil. Email: marceila@netsite.com.br
IINurse, Associate Professor of Maternal and Child Nursing and Public Health Department of Ribeirao Preto School of Nursing, University of Sao
Paulo. Ribeirao Preto, Sao Paulo, Brazil. Email: maclapis@eerp.usp.br
IIIStatistics, Associate Professor of Maternal and Child Nursing and Public Health Department of Ribeirao Preto School of Nursing, University of Sao Paulo.
Ribeirao Preto, Sao Paulo, Brazil. Email: cbsantos@eerp.usp.br
IVNurse, Doctorate Professor of Maternal and Child Nursing and Public Health Department of Ribeirao Preto School of Nursing, University of Sao Paulo.
Ribeirao Preto, Sao Paulo, Brazil. Email: thaisog@eerp.usp.br
VArticle extracted from the master thesis Preparation and validation of an instrument for identification of nursing practice related to the management and the prevention of radiodermatitis,
school of nursing of Ribeirao Preto, University of Sao Paulo, 2012.
DOI: http://dx.doi.org/10.12957/reuerj.2015.12677
ABSTRACT
This descriptive study conducted between January and September 2011 in Ribeirão Preto aimed to describe the basic methodological steps in constructing an instrument to identify nurse's practice in prevention and management of acute skin reactions caused by radiation. In the first step, important items to compose the instrument were identified and assembled into seven groups totaling 74 items. Once structured, the instrument was submitted to content and appearance validation by seven judges. The results of this stage showed that, overall, the instrument was considered clear, objective and well structured, an essential step in the validation process. A valid, reliable instrument will allow systematic collection of information and consequent identification of how care has been performed.
Keywords: Radiotherapy; radiodermatitis, professional practice; validation studies.
INTRODUCTION
Radiation therapy is an important modality for cancer treatment and, despite advances in radiation techniques, patients still experience adverse events. Among these are the acute skin reactions, known as radiodermatitis, characterized by erythema, pruritus, dry and wet peeling1.
Nursing care related to prevention and management of these reactionsV are inherent to the exempted care to cancer patients, as the nurse, by means of the nursing consultation performs actions such as: orientation to the patient about the action of radiation as well as on the care directed to the irradiated area to minimize adverse events, aimed at self-care; evaluation of the area and the identification of toxicity present in the irradiated tissues; prescribing the appropriate products according to the degree of the skin reaction2.
Measuring instruments aimed at identifying practical nurses make it possible to identify gaps in knowledge and difficulties encountered, such as non-use of institutional protocols and care based on myths and common sense instead of using scientific evidence.
The adoption of valid and reliable instruments to identify this practice is fundamental in that lends credibility to the information, which subsidize analysis able to provide improvements in care3, as the targeting of educational actions aimed at remedying the problems of professional practice, which are associated with quality of care.
Given the above, this study aimed to: to describe the basic methodological stages of building an instrument for the practice of identifying nurses in the prevention and management of radiodermatitis.
LITERATURE REVIEW
Aiming at the identification of tools used to assess the practice of health care professionals in prevention and management of radiodermatitis, a search of the scientific literature and identified some studies that evaluated the professional practice in this circumstance3-10. By analyzing the data collection form, observed: some7.10 used a questionnaire composed of open questions. Others3, 8, 9 used the mixed form, namely, questionnaires that had issues opened and closed. The construction of some instruments based on the literature review and expert opinion8.9 or literature review6.
In relation to the type of response of the instruments, the scale of Likert scale of four periods (always, sometimes, never)8,9 and dichotomous reply (yes/no)3,4.
Three studies reported the amount of items: 58 items8,9 and 13 items3. The items addressed a variety of aspects, such as written guidelines4; etiology of cutaneous reactions, incidence, intrinsic factors and extrinsic, instruments for assessment of skin reactions 7 and targeted care to prevention, erythema, desquamation and dry damp peeling, besides the use of topical agents3, 4, 6 -10.
It should be noted that there was no description of the instrument in one study5. The other instruments cited in other studies 3, 4, 6 -10, have not been subjected to the validation process, in addition to not having been found in the literature study that depicts the elaboration and validation process of an instrument to identify the practice of professionals in management and prevention of radiodermatitis.
Highlights include an author8 released the questionnaire in its entirety and authorized its use, however we opted for not applying it, because it has not been validated and also for having been directed to a different population proposed in this study.
METHODOLOGY
This is a descriptive study, which adopted the methodology adapted described by European projects Disabkids® and Kidscreen ®, whose proposal is described in other studies11,12 and was developed between the months of January to September 2011 in the city of Ribeirao Preto.
Went through four stages, however, the present study will describe only the first three stages.
The first stage consisted of a literature review and aimed to identify relevant items related to prevention and management of radiodermatitis.
Inclusion criteria adopted for the selection of the articles were: publication in its entirety online, in Portuguese, Spanish or English, publication date from January 1990 to July 2011 and theme prevention and management of radiodermatitis.
Nine studies met the inclusion criteria, but a reference was repeated in the databases Biomedical Database (EMBASE) and PubMed. Therefore, eightstudies3-10 were included in the analysis.
A systematic search was conducted in the Latin American literature and Caribbean Health Sciences (LILACS), Medical Literature Analysis and Retrieval System Online (MEDLINE), Cumulative Index to Nursing and Allied Health Literature(CINAHL), the digital library of theses and dissertations of the University of Sao Paulo (USP) and Google Scholar, with the following keywords: skin reactions, radiotherapy, radiodermatitis, management, to prove or disprove the materiality of items identified in the survey described above.
In the second stage the selection of relevant items and the development of the first version of the instrument.
In the third stage, the validation of content and appearance of the proposed instrument through the use of a questionnaire, composed of identification data and data related to the analysis of the instrument: clarity, how the items were grouped, coherence between them and their answers, objectivity, ease of reading and understanding.
There were 11 experts on oncology and validation tools invited to participate in this stage. Of these, one didn't accept participate and three did not return the assessment questionnaire within the time limit set. Thus, seven judges collaborated with the instrument validation process. A descriptive analysis of the data was performed.
The survey was developed on the basis of resolution No. 196/96/CNS/MS13 which ensures the Statement of Informed Consent. This project was approved by the Research Ethics Committee of Pius XII Foundation, according to the protocol under No. 442/2011.
RESULTS AND DISCUSSION
In the first step it was possible, through analysis of the available studies in the scientific literature, enumerate the guidelines and products used, and identify relevant items to prepare the measurement instrument.
Directed to radiodermatitis care identified were: written orientations3-5; use of scale for radiodermatitis evaluation6; wash and use of soap in the radiated area3-9 without friction and smooth4, 5, 7; use of topical agents3, 6, ,6,9,10; aloe vera 3; aqueous cream; and corn starch baby powder from Johnson's® 3, 8, 9; and glaxal lubriderm3; vaseline, E45, diprobase, epaderm, doublebase, cavilon cream or film10; avoid sunlight, direct heat in the area6.7 and extreme temperatures6; do not put hot water bottle and ice, not to go to the sauna, not exposed to the wind; wear loose-fitting clothes and cotton8 decrease friction in the treatment area6; do not use deodorant when irradiated region is the armpit3; Don't swim in chlorinated water7; do not use a tie8.
As for the products used in the presence of damp, peeling: gentian violet3-5.7 ,9; silver sulfadiazine3, 5, 8, 9; steroids such as hydrocortisone cream topics3, 9, 10 and5; betamethasone with Vaseline gauze7;6; solugel biafine, eosin , 4 3, 8, 9, neosporim3; talcum powder6, 8, 10; cleaning the wound with physiological solution8; 5%, iodine, kaltostat jelonet 4, use of occlusive coatings as polyurethane , hidrocoloide and non-adherent silicone, skin moisturizers8,9.
In the presence of erythema, the use of moisturizing creams7, 9, 10 and the sorbolone6; almond oil and lotion containing vitamin A and D; the 2% eosin a8; talcum powder7.8, cream containing antifungal and steroid topic10; avoid washing the area 9.
Considering patients with dry flaking: steroids topics4-6 as the hydrocortisone cream3; moisturizer8,9; corn starch5; talc4,8; almond oil8.9; silver sulfadiazine8; aloe vera4, 6, 9, containing vitamin E cream, pawpaw cream and mosquetas rose oil6; Chamomile4,9, Marigold, E454; avoid washing the irradiated area9; do not place ice packs and not washing the irradiated area8.
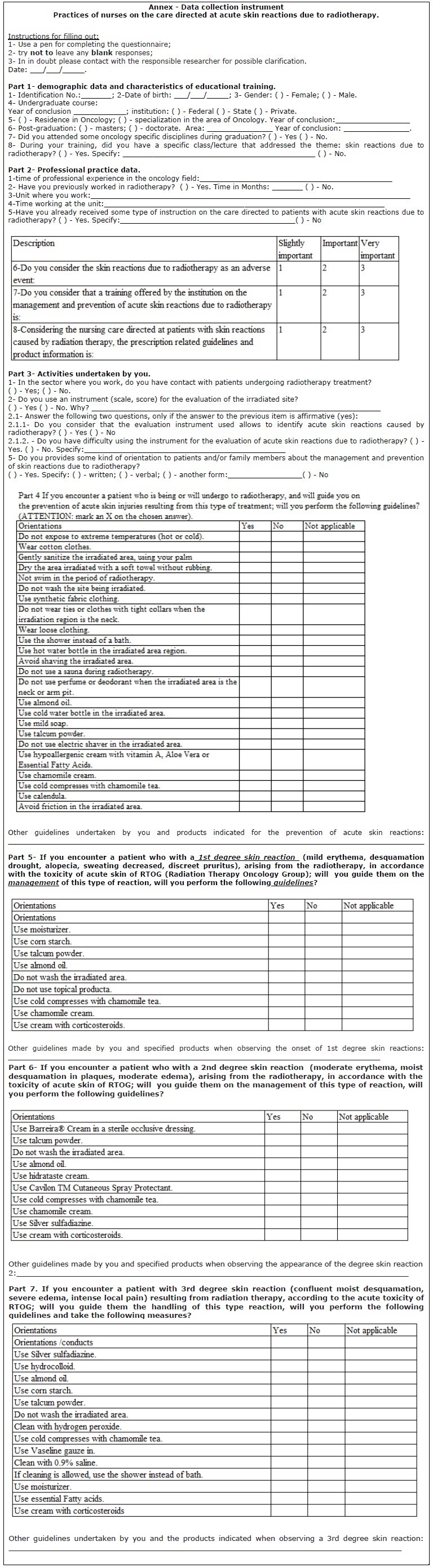
Products and guidance on the management and prevention of the relevant radiodermatitis were chosen to compose the final document presented in the Annex.
After selecting the items was held the grouping into seven parts, especially parts 4, 5, 6 and 7 of the instrument: guidelines and products related to the prevention of radiodermatitis with 25 items; management of skin reactions grade 1, characterized by light erythema, dry desquamation, alopecia, decreased sweating, slight pruritus with items 9; management of skin reactions grade 2, characterized by erythema and moderate swelling, moist desquamation in plates 10 items; management of grade 3 skin reactions, qualified by confluent moist desquamation, severe edema, intense local pain, with 14 items.
Part 1 was built with seven items related to demographics and characteristics of educational training, aimed at characterizing the group to which the instrument is intended; Part 2 with six items, includes the data of professional practice, and Part 3, with three items concerning the activities carried out by professionals.
After preparation, the first version of the instrument, with a total of 74 items, was assessed by seven judges. They all had at least a Masters degree, more than 10 years and 4 (57%) acted in assisting cancer patients in radiation treatment.
Content validation of the instrument, the judges considered him clear, objective and well structured. In relation to the answers attributed to each item, 5 (72%) agreed in parts. In order to improve understanding, they made some considerations described.
Part 1-demographics and characteristics of educational training: items 3 (graduation), 4 (residence or specialization) and 5 (graduate): specify whether they are general courses or in the area of Oncology; Insert the word course (item 3), institution where she completed her (items 4 and 5) and load time of the courses (item 4); replace the word type by course in item 5 (graduate); item 6 (for the realization of any discipline of Oncology during graduation): not to limit the period of graduation.
Part 2-the professional performance Data: include item on long experience in radiotherapy and the importance of prescription in relation to nursing care; item 4 (information received in health care services targeted to radiodermatitis): remove the term somehow and not to limit only the information received in the health institution; present the contents of the item 6 (linked with the importance attributed by the health professional radiodermatitis) before the content of the item 5 (importance attributed by the professional training on the management and prevention of skin reactions); replace the response average important category items by 5:06 important.
Part 3-activities carried out by professionals: item 2 (standardized instrument for the institution to evaluate the irradiated site): replace the expression the instrument by some instrument; change the options of answers, including: no, because the institution does not, although the institution has a protocol; changing the header of this part of activities performed by professionals, by activities conducted for you. Add other issues relating to the assessment tool of the irradiated site: If identifying the radiodermatitis and if there is any difficulty in use.
Parts 4, 5, 6 and 7-radiodermatitis prevention-related Guidelines, guidelines related to the management of skin reactions degree 1, 2 and 3 degree grade: write the guidelines with verbs in the infinitive; put a question mark at the end of the initial text and write in utterance acute toxicity of skin of the Radiation Therapy Oncology Group (RTOG). In relation to the open questions-further guidance and products listed when noted the appearance of skin lesion degree 1, 2 and 3, standardize further guidance, pipelines and products and added by you.
In relation to the category of responses (Yes, no) of 4 parts, 5, 6, 7, on the guidelines on prevention and management of radiodermatitis, add the category of response Yes and no, the category does not apply or do not know, because sometimes the participant fails to carry out the guidance, for not being a product standardization and not because I don't know; remove number 1 for Yes and 2 for not, leaving without number.
Replace the item use of cotton for use of cotton clothing. Change the item using hypoallergenic cream with vitamin A, aloe vera and Essential Fatty Acids (EFA) to use hypoallergenic cream with vitamin A, aloe vera or EFA. Remove the concentration of Chamomile cream. Include item using corticosteroid cream and avoid friction on irradiated area. Standardize the term acute reaction or injury. Add collection date in the header, and thank you for your cooperation or thank you for your participation in the end. Avoid sentences with or dividing them. Write in words the meaning of EFA. Remove Item use of any product, lotion or cream on the irradiated area, whereas there are other items that describe products, as talc powder, almond oil. Still on part 4, item-Sanitize radiated area gently, using the Palm of your hand: define which means soft in terms of hygiene.
The answers of the judges were analyzed individually, and considerations relevant to the instrument or population under study and consensus among them were identified. Such considerations subsidized changes that originated the instrument version, already presented.
The instrument is a questionnaire should be filled out by the nurse or a health professional and objective identify the actions taken by the professionals in the management and prevention of radiodermatitis.
A systematic assistance performed by nurses is essential and must include clinical evaluation, planning and implementation of the plan of care, appreciation of the conduct, which should be based on institutional protocols14 and on scientific evidence. Only in this way, this care often is specific and complex will include quality standards and ethical principles15. The use of instruments and evaluation questionnaires is a key resource in clinical practice, since it allows to identify the real needs and the necessary conditions for the realization of this systematic care 16.
CONCLUSION
The use of a valid and reliable instrument for quantitative measurement of this subjective construct will enable a systematic collection of data will enable to know the characteristics of care afforded to cancer patients during radiotherapy. Also, the degree of knowledge of professionals in this area, although specific, is crucial to a quality care.
To obtain this tool the following methodological stages were necessary; the bibliographic search in scientific literature, systematically and comprehensively enabled identifying the relevant items on the professional practice related to the prevention and management of this toxicity.
The assessment by experts was an important step in the validation process, since the judges proposed changes for a better understanding of the items of the instrument. However, it is essential to submit the instrument validation process semantics, because such steps complement each other.
Among the study's limitations: difficulty of understanding of the questionnaire used for content validation of the instrument by some judges; the identification of common products in clinical practice and that do not contemplate the reality of Brazil.
REFERENCES
1.Glover D, Harmer V. Radiotheray-induced skin reactions: assessment and management. Br J Nurs. 2014; 23: S28-5.
2.Blecha FP, Guedes MTS. Tratamento de radiodermatite no cliente oncológico: subsídios para a intervenção de enfermagem. Rev Bras Cancerol. 2006; 52:151-63.
3.Bolderston A. Skin Care recommendations during The radiotherapy: a survey of Canadian practice. Can J Med Radiat Technol. 2003; 34:3-11.
4.Lavery BA. Skin care during radiotherapy: a survey of UK practice. Clin Oncol. 1995;7:184-7.
5.Nystedt KE, Hill JE, Mitchell AM, Goodwin F, Rowe LA, Wong FLW, et al. The standardization of radiation skin care in British Columbia: a collaborative approach. Oncol Nurs Forum. 2005;32:1199-205.
6.Kumar S, Juresic E, Barton M, Shafiq J. Management of skin toxicity during radiation therapy: a review of the evidence. J Med Imaging Radiat Oncol. 2010;54:264-79.
7.Glean E, Edwards S, Faithfully S, Meredith C, Richards C, Smith M, et al. Intervention for acute radiotherapy induced skin reactions in cancer patients: the development of a clinical guideline recommended for use by the college of radiographers. J Radiother Pract. 2001;2:75-84.
8.D´haese S, Bate T, Claes S, Boone A, Vanvoorden V, Efficace F. Management of skin reactions during radiotherapy: a study of nursing practice. Eur J Cancer Care. 2005;14:28-42.
9.D´haese S, Roy MV, Bate T, Bijdekerke P, Hung VV. Management of skin reactions during radiotherapy in Flanders (Belgium): a study of nursing practice before and after the introduction of a skin care protocol. Eur J Oncol Nurs. 2010;14:367-72.
10.Hollinworth H, Mann L. Managing acute skin reactions to radiotherapy treatment. Nurs Stand. 2010;24:53-64.
11.Fegadolli C, Reis RA, Martins STA, Bullinger M, Santos CB. Adaptação do módulo genérico Disabkids® para crianças e adolescentes brasileiros com condições crônicas. Rev Bras Saúde Matern Infant. 2010;10:95-105.
12.Deon KC, Santos DMSS, Bullinger M, Santos CB. Translation and cultural adaptation of the Brazilian version of Disabkids® atopic dermatitis module (adm). Rev esc enferm USP [Scielo-Scientific Electronic Library Online]2011[cited in 2015 oct 01].45:441-7. Available from: http://www.scielo.br/pdf/reeusp/v45n2/v45n2a20.pdf.
13.Ministério da Saúde (Br). Conselho Nacional de Saúde. Resolução n°196, de 10 de outubro de 1996. Diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. [Internet] 1996 [citado em 01 out 2015]. Disponível em: http://www.datasus.gov.br/conselho/resol96/RES19696.htm.
14.Dantas DV, Torres GV, Dantas RAN. Assistência aos portadores de feridas: caracterização dos protocolos existentes no Brasil. Cienc Cuid Saude [Internet]. 2011 [citado em 01 out 2015]. 10:366-72. Disponível em: http://www.periodicos.uem.br/ojs/index.php/CiencCuidSaude/article/view/8572/pdf .
15. Rabeh SAN, Gonçalves MBB, Caliri MHL, Nogueira PC, Miyazaki MY. Construção e validação de um módulo educativo virtual para terapia tópica em feridas crônicas. Rev enferm UERJ. 2012;20:603-8.
16. Trotte LAC, Lima CFM, Pena TLN, Ferreira AMO, Caldas CP. Adaptação transcultural para o português do End of life comfort questionnaire-patient. Rev enferm UERJ. 2014;22:461-5.