RESEARCH ARTICLES
Exploratory study of patient safety measures at hospitals in Rio de Janeiro
Ruth Francisca Freitas de SouzaI; Lolita Dopico da SilvaII
IRN. M.Sc. student in the Graduate Program in Nursing of the School of Nursing of the Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil. Email: ruthffs@hotmail.com
IIPost-doctoral degree from Universidade Federal de Santa Catarina. Professor in the Graduate Program in Nursing of the School of Nursing of the Universidade do Estado do Rio de Janeiro – Rio de Janeiro, Brazil. Coordinator of the Intensive Nursing Course. Pro-scientist with scholarship at the Universidade do Estado do Rio de Janeiro. Email: lolita.dopico@gmail.com
ABSTRACT: The main aim of this exploratory study was to examine patient safety measures through information gathered from risk managers. The qualitative data analysis-based study was carried out at five hospitals in Rio de Janeiro City, from March to August 2013, by applying a semi-structured questionnaire to fourteen risk managers. All were found to participate in continued professional development activities. The activities least pursued were techno-, hemo- and pharmaco-vigilance (29%). They perform patient identification (100%), followed by clean care (86%), control of catheter-related blood infection (64%), and safe surgery (64%). Thus, the risk managers were all involved in patient identification. However, contrary to what is currently proposed, they implemented the measures that require less investment, and their actions were directed mainly to continued professional development.
Keywords: risk management; hospital; patient safety; nursing.
INTRODUCTION
Patient safety was defined by the World Health Organization (WHO) as the reduction in the risk of unnecessary harm associated with healthcare to a minimally acceptable level, where minimally acceptable relates to the current information, available resources and context in which the care is provided1.
Patient safety covers the risks involved in health care and seeks to minimize these risks, besides reducing or eliminating the Adverse Effects (ADEs), which are incidents that cause harm to patients1.
According to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO), the people responsible for disseminating and implementing the targets for patient safety and also for identifying, assessing and reducing the risk of harm being caused to patients are the risk managers2. The duties of the risk managers include the creation of a database of adverse events’ notification, the investigation of adverse events, the communication with the team and leaders in order to perform changes in accordance with the data found3.
Recently in Brazil, the National Program for Patient Safety (PNSP) was released, and it defines risk management as systematic and continuous application of policies, procedures, conducts and resources for the identification, analysis, assessment, communication and control of risks and adverse events that affect safety4.
From this perspective, an exploratory study was developed in order to analyze the initiatives developed to ensure patient safety, based on the information provided by the risk managers. The study can be justified based on the fact that, in the hospital context, the information, programs and targets are shown to be decontextualized from a larger set of information, in a way that large global campaigns are often not adopted in the hospitals.
LITERATURE REVIEW
In the 2000s, the security issues started to be the target of worldwide research, due to the release of the report To err is human: building a safety health system developed by the Committee on Quality of Health Care in America of the Institute of Medicine(IOM). This report presented impressive data from the American healthcare system, such as the fact that at least 44,000 to 98,000 people die in hospitals each year as a result of errors that could have been avoided5.
In that period, initiatives of various institutions around the world emerged, seeking to prevent adverse events and implement strategies to support patient safety in hospitals. For example, there are the six targets for patient safety presented by the JCAHO, and these were: identifying patients correctly; improving communication; improving safety related to risky medication; stopping surgeries being performed in the wrong body parts or patients; reducing the risk of having infections and reducing the risk of wounds resulting from bed falls6.
Similarly, the campaign of the five million lives by the Institute for Healthcare Improvement(IHI) can be mentioned, and this is composed of prevention initiatives of pressure ulcers; the reduction in the number of infections of Methicillin-Resistant Staphylococcus Aureus(MRSA); the prevention of harm resulting from Potentially Dangerous Medication (PDMs) (anticoagulants, sedatives, narcotics and insulin); the reduction of surgical complications; and the evidence-based care concerning heart failure7.
These safety measures started being required by the accrediting institutions, such as the JCAHO, the largest hospital accreditation institution in the world, which have demanded that the accredited organizations implement the six targets of patient safety since 20082.
In Brazil, risk management can be found in the scope of accredited hospitals and also in the sentinel hospital network of the National Health Surveillance Agency (ANVISA)8.
As previously mentioned, risk management in the accredited hospitals is aimed at identifying and analyzing risks, in order to reduce the occurrence of adverse events. However, according to the ANVISA, risk management has a health risk duty, with the purpose of detecting problems in the products under surveillance that compromise the quality and safety of their use and sending reports to health authorities about these problems8.
According to the ANVISA, the risk manager is responsible for coordinating the health risk management team in hospitals of the healthcare service and articulating the care in three major areas: pharmaco, techno and hemo surveillance8.
More recently, due to the increase in the discussions about patient safety and strategies to reduce adverse events, a new definition of the profile of risk managers, with their actions also focused on patient safety, have emerged3,4.
METHODOLOGY
This is an exploratory study, carried out between March and August 2013, in five hospitals located in the city of Rio de Janeiro, all of them part of the sentinel network or accredited, where the presence of a risk manager is compulsory.
The population was composed of 14 risk managers, who met the following selection criteria: to perform tasks related to patient safety; to have been performing this role for at least six months and to accept participating in the study through signing the Informed Consent Form. There were no refusals to participate in the study. A semi structured questionnaire was used as the data collection technique. The data were stored in an electronic spreadsheet using the program Microsoft® Excel 2010. The present study complied with the Rules of Research involving Human Beings, as per Resolution number 466/2012 of the National Health Council9, and was submitted and approved by the Research Ethics Committee (REC), opinion number 292,752/2013.
RESULTS AND DISCUSSION
The study population was mostly composed of nurses, 13 (93%), women, 14 (100%), with average age of 45 (±4), who graduated 20 years ago (±6.9) and have been in the professional practice for almost the same amount of time, 18 years (±7.1), 50% who have completed a Master’s degree but most of them, 12 (86%) did not have a specific management education.
All risk managers reported to have their activities focused on continuous education, and the least performed actions are those related to the analysis of ADEs, 7 (50%) and the techo, hemo and phamaco surveillance actions, 4 (29%).
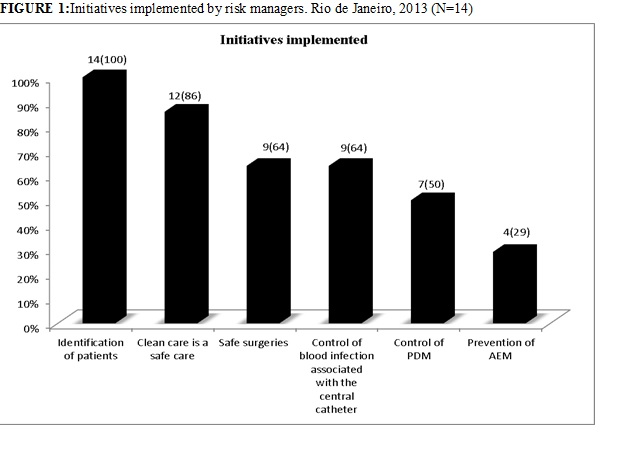
The answers related to some of the global patient safety targets of the JCAHO, WHO and IHI show that all of them implement the identification of patients, 14 (100%), followed by the clean care, 12 (86%), control of blood infection associated with the catheter, 9 (64%) and safe surgery, 9 (64%). The least implemented initiatives were related to the control of PDMs, 7 (50%) and prevention of adverse events caused by medication, 4 (29%), as shown in Figure 1.

FIGURE 1: Initiatives implemented by risk managers. Rio de Janeiro, 2013 (N=14)
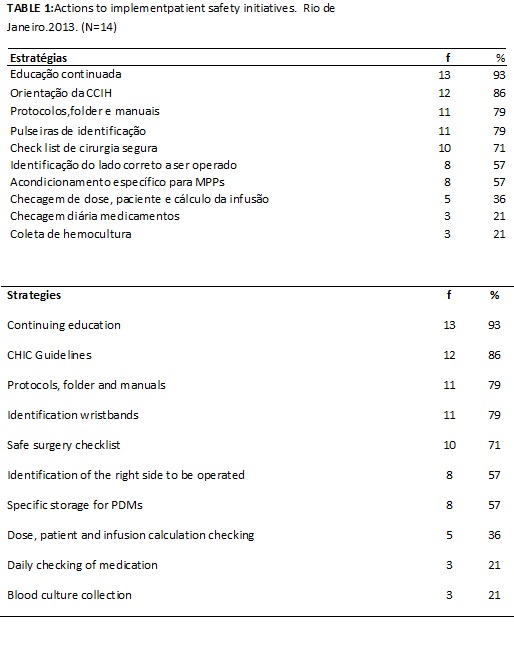
In order to implement the initiatives involving patient identification, clean care and others, the managers informed to develop the following institutional actions: courses through continuous education, 13 (93%), guidelines from the Commission of Hospital Infections Control (CHIC), 12 (86%), development of protocols, folders and manuals, 11 (79%), and checklist of safe surgery, 10 (71%). The daily checks for potentially dangerous medication and collection of blood culture were the least performed actions, 3 (21%), as shown in Table 1.
All risk managers informed that they use continuous education as a strategy to disseminate the programs. In contrast, only half of them perform the monitoring of adverse events, and the implementation of ADEs monitoring systems is currently one of the most used tools concerning risk management10.
Another fact highlighted in this study relates to the reduced number of risk managers who perform actions recommended by the ANVISA.
According to the ANVISA, the health risk manager should develop and encourage health surveillance actions in hospitals; help identify, investigate and send the notification of events, or technical complaints associated to medication, blood and blood products, equipment and articles of medical use; coordinate the actions required in the techno, phamaco and hemosurveillance, among others8.
Recently, on 1 April 2013, the PNSP was released through Rule number 529/2013, with its actions instituted by the RDC number 36, of 25 July 2013, which addresses the importance of risk management and a Patient Safety Unit (NSP), involving professionals focused on detecting the adverse events and implementing the programs of the Ministry of Health for patient safety. The NSP is defined as the body of the healthcare service established to promote and support the implementation of actions focused on patient safety4.
This shows a significant change of concepts and actions focused on patient safety that did not yet exist; however it is a recent movement of changes and understanding about patient safety and, in addition to new strategies, the involvement of all the professionals focused on a safety culture is required, with an awareness about the importance of patient safety and changes in behaviors and values11.
The results showed that most risk managers implement the initiatives of identification of patients, clean care, control of blood infection associated with the catheter and safe surgery, all of which will be discussed below.
Identification of patients
All risk managers implement the identification of patients, which is consistent with the proposed reduction of adverse events related to the identification of patients, since it is an important strategy for a safer care, with reduction of errors such as the blood transfusion on the wrong patient, wrong medication, wrong procedure and baby swap12.
Of all the risk managers involved the study, 11 (79%) of them state using the wristband as a strategy for patient identification. This is a large number when compared to a study carried out in 2009, involving 389 hospitals located in eight countries of Europe, where the authors concluded that, of the strategies observed, the least performed one in the hospitals was the use of wristbands for identification of adults, with only 25% of identification in medical center units, 29% in surgical units and 47% in maternity hospitals13.
The JACHO6 and WHO12 designed campaigns such as the international targets for patient safety and the nine solutions for patient safety, which address the use of identification wristbands as the most disseminated method so far, and also point out to the correct way of identifying patients.
Among the international targets for patient safety, the identification of patients is the first proposed target and its main measures are: to emphasize the responsibility of healthcare professionals to check the identification of patients before performing care actions, to identify patients through at least two identifiers, for example, name of patients and date of birth; to implement protocols to identify patients with the same name, in comma or confused; to encourage patients to participate in all the stages of the process, among others6.
Clean care is a safe care
The other initiative mentioned by most managers was the implementation of the program Clean care is a safe care. They report seeking to develop their guidelines through continuous education courses, in conjunction with folders and manuals.
The current priority of the campaign Clean care is a safe care is the multimodal strategy of hand washing6,12, which was not mentioned by any risk manager.
The multimodal strategy is focused on the study of University Hospitals of Geneva, based on the observation of hand washing, with an increase in hand washing from 48% to 66%, and in this period, the reduction of hospital infections from 16.9% to 9.8% and the MRSA rates reduced by half14.
A multimodal strategy included the continuous monitoring of the adherence to hand washing and feedback to professionals, as well as communication and education tools, reminders in the workplace and multiprofessional support in various levels of the institution14.
In 2007, the WHO and JACHO reinforced the guidelines for patient safety through the Solutions for Patient Safety, and its main orientations are: to provide easily accessed alcohol based sanitary hand lotions, the products should be at a distance equivalent to an arm’s length from where the care is being provided; to have access to a clean and continuous water source in all the taps and to have the facilities required to perform hand washing, as well as to show reminders that promote hand washing at the workplace3,12.
The ANVISA, when adhering to the first global challenge related to patient safety, through the campaign Clean care is a safe care, establishes five moments of hand washing12, as per Figure 2.
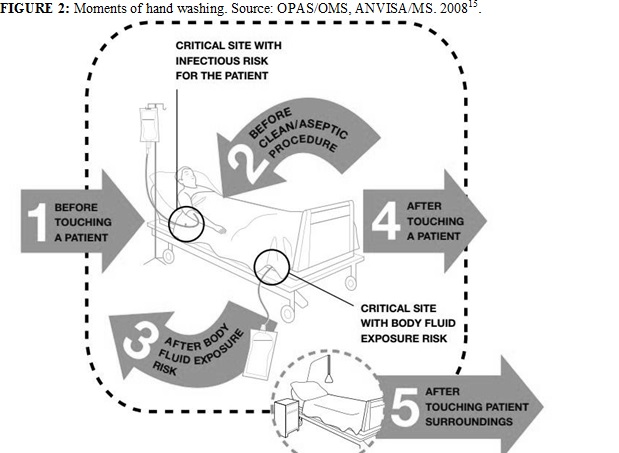
FIGURE 2: Moments of hand washing. Source: OPAS/OMS, ANVISA/MS. 200815.
Control of blood infection associated with the central catheter
The main initiative to control blood infection associated with the catheter is performed by the IHI and is part of the campaign called Campaign of the 100,000 lives. Its prevention guideline is the implementation of a bundle of a venous central catheter, which consists of a group of interventions for patients with a central intravascular catheter that, when jointly implemented, result in better results than when individually applied7.
The bundle has five main components: hand washing; maximum precautions barriers during the insertion of the catheter; skin antisepsis using Chlorhexidine; selection of the best place to insert the catheter, preferably into the subclavian vein; daily review of the need for the catheter, with immediate removal of unnecessary catheters7.
A fact that draws attention in this study is that 9 (64%) managers reported to develop the infection control program related to central venous catheter, but the implementation was limited to the monitoring of the CCIH, through blood culture results and not necessarily through the application of the globally used bundle proposed by the IHI.
Safe surgeries save lives
In this study, 9 (64%) risk managers state to implement the campaign Safe surgeries save lives and, for this, their main actions are the application of the safe surgery checklist, 11 (71%) , and the identification of the correct side to be operated, 8 (57%).
The fact that a large number of the risk managers use the surgery checklist shows consistency with the measures for prevention of adverse events, since the checklist is the main tool of the WHO12 and the JACHO6 to ensure that surgeries are safe.
The checklist consists of a check list to be performed in three stages: before the anesthetic induction (sign in), before the surgery (time out) and before the patient leaves the operating room (sign out) and the following main issues to be checked are: to identify the patient, to mark the surgical site, to verify if the patient has any allergies, to confirm that all teams members are introduced by their names, to check if the count of surgical instruments (swabs and needles) is correct, among others12.
In a longitudinal study conducted in 2009 in eight European hospitals about the effect of the safe surgery checklist, the authors found a reduction in the perioperative mortality and morbidity. According to them, the mortality rate was 1.5% before the introduction of the checklist and it was reduced to 0.8% after its implementation, similarly to the postoperative complications which were reduced from 11.0% in the beginning of the study to 7.0% after the introduction of the checklist16.
Therefore, the fact that most risk managers implement the safe surgery checklist shows a great advancement in relation to patient safety in the researched institutions.
Control of potentially dangerous medication and prevention of adverse events involving medication
The PDMs control and the prevention of adverse events related to medication (AEM) were the least mentioned initiatives by the risk managers. However, the errors and the AEM happen daily in patient care and, according to the IOM, the AEM are responsible for 7,000 deaths, most of them being avoidable5.
The PDMs are medications most likely to cause significant harm to patients, even when they are correctly used. Among them, the anticoagulants, sedatives, narcotics, insulin and others can be mentioned, and the list is made available in Brazil by the Safe Medication Practices Institute (ISMP)7,17.
Among the recommendations for control of PDMs is the dissemination of the list of PDMs, the number of presentations and concentrations of PDMs, which must be limited. Stocking these medications in the healthcare units should be avoided, and the dosages of these medications should be double checked, among others18.
There are several guidelines to prevent the AEM. Among them, there are the medical prescription guidelines, for example, the prescriptions must be legible, complete and have no abbreviations. It is also recommended to use the generic name of the medications and avoid verbal prescriptions. Other guidelines are related to dispensation, such as storing them in separate and different places and in alphabetical order. There are guidelines involving the administration of medication, such as double checking the medication and the nine rights before administering any medication to a patient (right patient, right medication, right dose, right route of admission, right time, medication compatibility, right orientation to patients, right to refuse the medication and right recording), among others18.
Furthermore, it is important that professionals involved in the prescription, dispensation and administration of medication have an extensive knowledge and specific skills about pharmacology, drug interactions and reactions to the drugs used, in order to avoid AEMs19.
However, the risk managers participating in the study mentioned only three of the many recommendations to control the PDMs and prevent AEM, and these were the specific storage for the PDMs, 8 (57%), checking of dose, patient and infusion calculation, 5 (36%) and daily checking of medications, 3 (21%). This shows that little has been developed compared to the large volume of information that current exists about the issue.
CONCLUSION
Through the study, it was possible to note which of the globally proposed initiatives have been implemented by the risk managers in some hospitals in Rio de Janeiro. This information is important to identify the necessary changes to increase the actions focused on patient safety.
It was found that, most of all the initiatives mentioned are performed by them, which shows that they are in line with what is currently recommended, especially in relation to the identification of patients raised by all the risk managers, the hand washing raised by 86% of them and the use of the safe surgery checklist, raised by 71% of them.
It is possible to note that the main initiatives developed by the risk managers are those most disseminated until now by the leading institutions in the area of patient safety, and are also the initiatives that basically depend on continuous education in order to be put into practice, without the need for greater investments.
The fact that the initiatives related to the medications have been the least raised by the risk managers shows the need for further investments in this area, since it is a major problem of public health, which is common in hospitals and easy to be resolved with behavioral changes.
Moreover, in addressing the actions required to put the initiatives for patient safety into practice, the risk managers rarely mention the numerous strategies prescribed in the current literature.
The risk managers report performing duties mainly related to continuous education. However, the main responsibility of the risk manager is the identification and reduction of risks to patients through the search for and the monitoring of adverse events.
The National Program of Patient Safety, recently released by the ANVISA, lays down the basis for a uniformity of concepts and actions for the implementation of strategies disseminated worldwide that, until then, did not exist in the healthcare policy of the country and this is an essential initiative to guide and demand that healthcare institutions seek to provide a safer care.
In addition, this program determines a group of professionals with duties that involve the investigation and analysis of adverse events, the implementation of patient safety protocols, and others previously mentioned, which until then were not recommended by the Ministry of Health and performed separately by risk managers in some healthcare institutions.
It is believed that the study helped identify some of the actions that have been performed for patient safety. It also contributed to the dissemination of initiatives for patient safety, as well as it emphasizes the importance of risk managers in this context and their duties, highlighting the need for training the professionals involved in risk management and the involvement of healthcare professionals in the campaigns and initiatives for patient safety, so that they are not applied in isolation but inserted into a safety culture.
The limitation of the study is that the research involved only 14 risk managers and five hospitals of Rio de Janeiro, which restricts the results in a way that they do not represent the hospital environment. Furthermore, it was a study that involved the completion of a questionnaire and may be subject to all the biases previously envisaged, such as memory lapses, incomplete information, among others.
Therefore, it is suggested that observational studies about the issue or exploratory studies involving a representative sample are conducted in order to investigate the reality of Brazilian hospitals in relation to patient safety measures currently adopted.
REFERENCES
1. World Health Organization. The conceptual framework for the international classification for patient version 1.1. Genebra (Swi): WHO; 2009.
2. Joint Commission on Accreditation of Healthcare Organizations. Manual internacional de padrões de acreditação hospitalar. 3a ed. Estados Unidos: Joint Commission Resources; 2007.
3. Boothman RC, Blackwel AC. Integrating risk management and patient safety. Clinical obgyn [Scielo-Scientific Electronic Library Online] 2010 [citado em 05 jul 2013]. 53:576–85. Available at: http:// www.clinicalobgyn.com.
4. Ministério da Saúde (Br). Portaria nº 529, de 1º de abril de 2013. Institui o Programa Nacional de Segurança do Paciente. Brasília (DF): Ministério da Saúde; 2013.
5. Institute of Medicine. Committee on Quality of Health Care in America. To Err is Human. Washington (DC): National Academy Press; 2000.
6. Joint Commission on Accreditation of Healthcare Organizations [site de Internet]. International Patient Safety Goals. [citado em 05 jan 2013]. Disponível em: http:// www.jointcommission.org/ international patient safety goals.
7. Institute for Healthcare Improvement [site de Internet].Overview of the 5 Million Lives Campaign. [citado em 05 jan]. Available at: http://www.ihi.org/IHI/Programs/Campaign/Campaign.htm?TabId=1
8. Agência Nacional de Vigilância Sanitária. [site de Internet]. Apresenta informações sobre gerência de risco. [citado em 5 feb 2013]. Available at: http://www.anvisa.gov.br/servicosaude/hsentinela/gerente_risco.htm
9. Ministério da Saúde (Br). Resolução nº 466, de 12 de dezembro de 2012. Brasília (DF): Ministério da Saúde; 2012.
10. Briner M, Kessler O, Pfeiffer Y, Wehner T, Manser T. Assessing hospitals' clinical risk management: Development of a monitoring instrument. BMC Health [Scielo-Scientific Electronic Library Online] 2010 [citado em 25 jul 2013]. 10: 337. Available at: http//: www-biomedcentral.com.
11. Silva LD. Segurança e qualidade nos hospitais brasileiros Rev. enferm. UERJ. 2013; 21:425-6.
12. World Health Organization. Collaborating [site de Internet]. Centre for Patient Safety- Patient safety solutions. [citado em 15 jan 2013]. Available at: http://www.ccforpatientsafety.org/patient-safety-solutions
13. Suñol R. Implementation of patient safety strategies in European hospitals. Qual Saf Health Care [Scielo-Scientific Electronic Library Online] 2009 [citado em 25 ago 2013]. 18: 57–61. Available at: http//: http://qualitysafety.bmj.com/content/18/Suppl_1
14. Pitet D, Huggonnet S, Harbarth S, Mourouga P, Sauvan V, Touveneau S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet [Scielo-Scientific Electronic Library Online] 2000 [citado em 25 ago 2013]. 356:1307-12. Available at: http//:www.ncbi.nlm.nih.gov/pubmed/11073019
15. Ministério da Saúde (Br). Organização Pan-Americana da Saúde. Agência Nacional de Vigilância Sanitária. Manual para Observadores. Brasília (DF): OPAS; 2008.
16. Haynes AB, Weiser TG, Berry WR. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med [Scielo-Scientific Electronic Library Online] 2009 [citado em 25 ago 2013]. 360:491–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19144931
17. Instituto para Práticas Seguras no uso de Medicamentos [site de Internet]. Lista de medicamentos potencialmente perigosos. [citado em 05 set 2013]. Available at: http://www.ismp-brasil.org/faq/medicamentos_potencialmente_perigosos.php
18. Ministério da Saúde (Br). Protocolo de segurança na prescrição, uso e administração de medicamentos. Protocolo integrante do Programa Nacional de Segurança do Paciente. Brasília(DF): Ministério da Saúde; 2013.
19. Silva LD, Santos MM. Interações medicamentosas em unidade de terapia intensiva:
uma revisão que fundamenta o cuidado do enfermeiro. Rev enferm UERJ. 2011; 19:134-9.