(a) Pearson Qui Square test;
(b) Fisher Exact test.
FIGURE 1: Number of positive cultures according to colleting time and localization of sample collection. São Paulo, June 2013.
ORIGINAL RESEARCH
Contamination of alcohol preparations for hand hygiene in a pediatric intensive care unit
Denise Miyuki KusaharaI; Ariane Ferreira Machado Avelar II; Agda Vinagre BragaIII; Maria Teresa de Melo MendesIV; Maria Angélica Sorgini PeterliniV; Mavilde da Luz Gonçalves PedreiraVI
I
Nurse, Science PhD, Department of Pediatric Nursing. Paulista School of
Nursing. Federal University of São Paulo, 2014. Brazil. Email: dkusahara@unifesp.br
II
Nurse, Science PhD, Associate Professor, Department of Pediatric Nursing.
Paulista School of Nursing. Federal University of São Paulo, 2014. Brazil.
Email: ariane.machado@unifesp.br
III
Pharmaceutical Biochemistry. Supervisor of Central Laboratory of the
Hospital São Paulo. Federal University of São Paulo, 2014. Brazil. Email: agdabraga@yahoo.com.br
IV
Nurse Master of Post-graduation Program Nursing Paulista School of Nursing.
Federal University of São Paulo, 2014. Brazil. Email: teetimelo@hotmail.com
V
PhD in Nursing. Associate Professor, Department of Pediatric Nursing.
Paulista School of Nursing. Federal University of São Paulo, 2014. Brazil.
Email: maria.angelica@unifesp.br
VI
PhD in Nursing. Associate Professor, Department of Pediatric Nursing.
Paulista School of Nursing. Federal University of São Paulo, 2014. Brazil.
Email: mpedreira@unifesp.br
VII
Study from the research project:
Practice hand hygiene determining factors, adhesion and promotion
strategies.
DOI: http://dx.doi.org/10.12957/reuerj.2016.10640
ABSTRACT
Objective: to ascertain microbiological contamination of alcohol gel bottles used for hand hygiene in a pediatric intensive care unit (PICU), by bottle location and time installed in bed. Method: exploratory study and microbiological analysis of 140 samples from lid, spout and internal content of alcohol gel bottles installed on PICU beds at a university hospital in São Paulo. Data was collected from May to June 2013, after approval by the Research Ethics Committee (No. 0440/11). Results: of the cultures, 54 (38.6%) showed bacterial growth, predominantly on the lid at all sampling times. The species identified were Staphylococcus coagulase-negative, Pseudomonas aeruginosa and Acinetobacter baumannii. Conclusion: alcohol gel bottles can become colonized during use in inpatient units and should receive special attention as regards the cleaning process.
Keywords: Hand hygiene; patient safety; intensive care units, pediatric; pediatric nursing.
INTRODUCTION
Hand hygiene is a relevant intervention for the control of infections related to health care (IRAS), considered one of the pillars to prevent infection in health services. However, even only a part of one simple implementation, the correct and consistent execution of this practice in the daily routine of health professionals still encounters resistance 1.
Given this context, one of the strategies used to promote increased adherence to hand hygiene practices in pediatric intensive care units (PICU) relates to the installation of alcohol gel bottles at the head and foot of the beds so that they are located exactly at the patient care area, allowing the professional easy access to them without having to move away from the bedside.
The key factor to be considered for the use of this kind of products in health services, is their antimicrobial efficacy. However, factors as the correct execution of techniques and acceptability to products may interfere with hand hygiene. Requiring manufacturers indicate volume and product application and time on labels is also needed to obtain more credibility to alcohol gels sold in Brazil2.
Alcohol gel bottles are routinely installed at the time of child admission to the PICU, remaining in place until the end of the product or child's move to another hospital. However, some children remain for a short period of time in the unit because they are indicated to stay in the PICU, as in some post-operative situations. Where this is the case, at the time of dismissal of the child, the alcohol gel bottles may contain large quantities of the product, so that one may ask from professionals if the same bottle can be subjected to cleaning and reused for another patient in another bed, or should be neglected because of the risk of contamination by microorganisms present in the child care area.
To try to remedy doubts arising from practice, we developed this study VII with the objective to determine the microbiological contamination of alcohol gel bottles used in a PICU, according to local bottle and length of stay in bed.
LITERATURE REVIEW
The IRAS are events that affect patients during the care process in hospitals or other health care services, being caused by infectious agents that were not present or incubated at the time of admission. It affects millions of people around the world and is considered one of the greatest global challenges for patient safety3. In addition, factors associated with scarcity and qualification of human resources, combined with inadequate physical structure of health services and the lack of HAI control measures contribute to this scenario4.
To prevent this type of infection and the spread of micro-organisms in the hospital environment, various practices are recommended as the use of antiseptics, applied to living tissue, as in hygiene, and antimicrobial agents, disinfectants used for the disinfection of surfaces, furniture and articles for hospital use. These agents are formulated with active ingredients of physical, chemical or combined nature, providing them with microbial no-spoilage and spoilage effects for microorganisms, and should be subjected to a quality control to establish, identify and quantify its antimicrobial activity, the principle assets of its formulation and its toxicity5.
Alcoholic preparations are one of the antiseptics classes widely used in health care services for hand hygiene. The alcoholic preparation for hand hygiene in gel and foam must contain alcohol in the f minimum concentration of 70%. with proven antibacterial activity in in-vitro laboratory tests (suspension test) or live tests, to reduce the number of microorganisms6.
The antiseptic rubbing hands with an alcohol preparation is characterized by applying the product in hand to reduce the load of microorganisms without the need for rinsing with water and drying with a paper towel or other inputs. It is a fast and effective means to inactivate potentially harmful microorganisms present on the hands, thus constituting one of the main forms of routine hand antisepsis3.5.
One of the factors influencing adherence to the professional hand hygiene concerns infrastructure practices such as, for example, the availability and access to resources needed to complete the procedure, like sinks, soap and alcoholic preparations. According to the Board Resolution number 42, of 25 October 2010, the National Health Surveillance Agency (ANVISA), the availability of alcoholic preparation for hand hygiene at the edge of the patient's bed is mandatory, so that the health professionals do not need to leave the place of care and treatment for hand hygiene, should the preparation be in a visible place and easy to access3, 6.
It is known that the degradation of compounds such as alcohol, may be caused by chemical, physical or microorganisms that may be present in various products and environments. Its presence can cause situations such as more rapid deterioration of the compounds, the product is inefficient, the user infections, among others. Thus, to evaluate the microbiological quality of the product is important to verify that the manufacturing procedures, storage, marketing and use of this product are adequate7.
Researchers evaluated the antimicrobial efficacy of antiseptics based products polyvidone iodine, chlorhexidine digluconate, benzalkonium chloride, triclosan and ethyl alcohol 35% and 70% by strains of Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli and Candida albican. At the end of the tests, it was observed that samples based on 35% ethanol and chlorhexidine digluconate were unsatisfactory for all strains of reference microorganisms. This result is a worrying factor because solutions containing chlorhexidine are widely used in hospitals as an antiseptic of choice for clinical and surgical procedures8.
Contaminated antiseptics can constitute a frequent source of microorganisms involved in outbreaks of infections in hospitals. Several situations can change the quality of alcohol and other solutions used in health services such as: raw material with different concentrations indicated, the not purified water used for dilution, storage in damp places and high temperature, packaging that do not protect extravasation, chemical or biological contamination by contact with the environment or with the hands, and routines that do not meet the technical best practice in the handling of these products9.
METHODOLOGY
An exploratory study by microbiological testing for contamination of alcohol gel bottles used for hand hygiene professionals and family in a PICU. The study was approved by the Ethics Committee of the Federal University of São Paulo (UNIFESP) under approval number 0440/11. After approval, there was the collection of data in a PICU of a university hospital of 630 beds, located in the city of São Paulo. The PICU has nine beds for care of newborns to adolescents under 18 years, affected by clinical or surgical diseases from other pediatric care units of the hospital as ready child relief, inpatient units and outpatient clinics, as well as transferred to other centers of health care.
In this study we adopted a non-probabilistic and convenience purpose sample consisting of 140 cultures obtained from nine bottles of alcohol gel, installed in the nine beds in the PICU.
Data collection began in May and ended in June 2013, being held by researchers, students and biochemical pharmaceutical study participants. Bottles containing alcohol gel 250ml were installed on a device attached to the back of the grid head and the lower grade of the PICU cots and submitted to the collection of samples for microbiological evaluation.
Four bottle sites were selected for the collection of samples for microbiological culture being: output end, cover, hose and gel. The samples were taken immediately after the removal of the bottle packaging box provided by the manufacturer and installation of the same in the child's crib, with no prior bottle manipulation, being called Control Culture. After we started using cultures, we obtained samples after 24 hours and after 48 hours of installation and completion of the content of alcohol gel in the vial or upon dismissal of the child from PICU.
Of the nine installed bottles, eight were included in the proposed microbiological analysis and one was discarded during the child's transfer to another unit before collecting the final sample.
To collect samples of the cover, outlet tip and hose was used swab sterile rubbed on the surface of each location. After the frictionswab It was dipped in a sterile tube containing broth Brain Heart Infusion (BHI).
The collection of alcohol gel was performed with a sterile Pasteur pipette, and 01 ml of this gel was aspirated in the center of the bottle, and mixed with 09 ml of broth Brain Heart Infusion (BHI), contained in a sterile tube. The solution was then homogenized 1:10.
After collection, the samples were transported in a thermal carrying case and immediately sent to the laboratory specialized in microbiology belonging to the institution where the study was developed. In the laboratory, the samples were incubated at 35 ± 2 ° C and then daily readings were held at 24h, 48h and up to 7 days of incubation, checking for turbidity of the broth.
In case of turbidity, the broth was held inoculation in blood agar with a sterile handle for exhaustion. The isolated bacteria were identified according to the manual use of standardized or automated Phoenix®.
The data obtained in the study of selected variables were stored in an electronic database, subject to the tab in the spreadsheet program Microsoft Excel ® and later analyzed.
Descriptive statistics were used, and qualitative variables represented by absolute frequency (f) and relative (%). To check the association between the variables we used Pearson's chi-square and Fisher exact, establishing the level of significance of 5%.
RESULTS AND DISCUSSION
We held 140 cultures in nine bottles of alcohol gel according to location, point and time of the collection. From these cultures, 36 were Culture Control (9 cover; 9 Output Tip; 9 hose, 9 gel); 36 cultures were 24 (9 cover; 9 Output Tip; 9 hose, 9 gel); 36 were crops of 48h (9 cover; 9 Output Tip; 9 hose, 9 gel); and 32 were End Culture of alcohol gel (8 cover; 8 Output Tip; 8 Hose, 8 Gel);
Of the 140 cultures examined, 54 (38.6%) showed bacterial growth, of which 17 (31.5%) were control cultures; 13 (24.1%) were culture 24 hours; 13 (24.1%) were cultures in 48 hours and 11 (20.3%) were culture bottle finish.
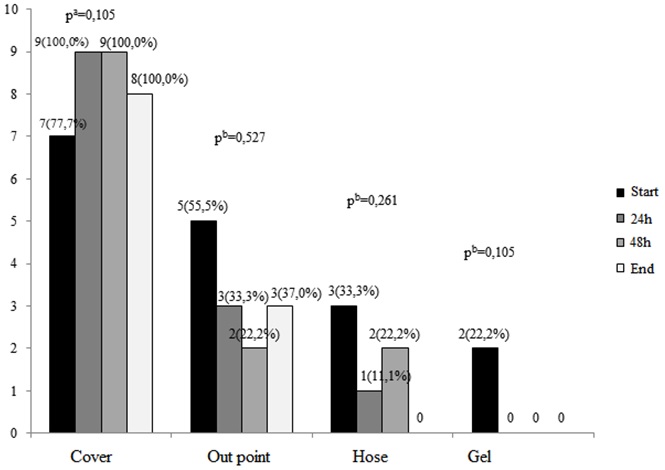
It was observed that the cover is where most identify bacterial growth takes place at all sampling times, and with advancing time, also on the cover an increase in the number of positive cultures can be observed. This fact was not observed other sites in the obtaining samples. However, this difference in colonization among the collection times was not statistically significant for any of the sites observed, as shown in Figure 1.

(a)
Pearson Qui Square test;
(b)
Fisher Exact test.
FIGURE 1: Number of positive cultures according to colleting time and localization
of sample collection. São Paulo, June 2013.
There were significant differences in the identification of Gram+ (p <0.001) and no growth (p <0.001) between the two locations. The details of the statistical analysis show that there was a growth of Gram+ significantly higher bacteria between cover and output point (p <0.001), cover and hose (p <0.001), cover and gel (p <0.001), and output point and gel (p = 0.001). In this respect the comparison between hose and gel (p = 0.078) showed no statistically significant difference and the comparison between output tip and hose proved marginally significant (p = 0.056). See Table 1.
TABLE 1:
Results of cultures by local of sample acquisition. São Paulo, June 2013.
(N=35)
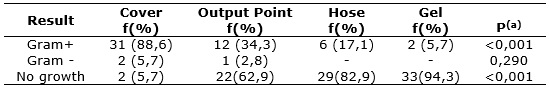
(*)Fisher Exact test
On the cover, there was an increase in the identification of Gram+ bacteria in the first 48 hours of study, which was not observed in other local gathering cultures. There was an isolation of Gram- bacteria in smaller proportions with no identifiable pattern to this growth. The absence of growth was observed in the output point and gel according to Table 2.
TABLE 2:
Results of cultures by local and time of sample acquisition. São Paulo,
June 2013.
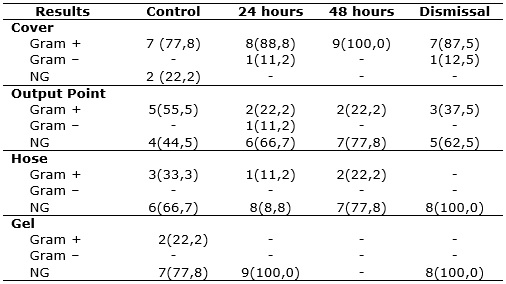
(*)
NG = No Growth
The results presented in Tables 1 and 2 show the predominance of Gram+ bacteria. According to primary data, of the 51 isolated species, 50 (98.0%) were Staphylococcus coagulase-negative and 1 (2%) speciesBacillus licheniformis. Isolated Gram bacteria species were 2Pseudomonas aeruginosa and one kind of Acinetobacter baumannii.
The PICU in which the research was carried out is characterized by a high incidence of infections related to health care caused by Gram- bacteria. Thus, a colonization by bacteria of bottles of this nature was expected. However, the results showed the majority presence of Gram+ bacteria. We isolated two kinds of Gram+ : the Bacillus licheniformis, which has little clinical importance because of their non-pathogenic nature, being widely distributed in nature, present in the soil, whose spores are spread with dust10.
The other isolated bacteria was the SStaphylococcus coagulant negative. There are about 33 species Staphylococcus coagulant negative known, of which the most common are:Staphylococcus epidermidis, Staphylococcus saprophyticus and Staphylococcus haemolyticus. They are usually identified in human skin and slime and have high potential for pathogenicity in neonates and immune compromised patients11. It should be noted that the place with the highest number of positive cultures was the alcohol gel bottle cap, where there is direct contact with the hands of staff members and family.
In this context, another question related to contamination of the hands of professionals after handling the bottle cap came up. Would a staff member triggering the bottle cap for alcohol gel dispensation, at this time get the hands colonized by the microorganism that was there? Can one have any clinical significance to the patient resulting from this? Or will the alcohol gel applied to the surface of the hands of the professional at this point be enough to inactivate these bacteria?
The cover colonization pattern presented is similar to the other locations. On the cover there were fewer colonized samples at the time of installation bottle in the child's bed, such colonization over time has increased, differing from other places where there was a trend to reduced colonization. One can hypothesize that this happens because antimicrobial action of alcohol gel. At the time of installation there has not been contact yet between alcohol, hose and output tip and these last two present some kind of settlement arising from the manufacturing and storage process.
However, after installation and initial use of alcohol, when the alcohol pumping mechanism is triggered, it starts to take the hose and the point of departure and probably its inhibition of bacterial growth effect starts to also prevail in these places, explaining the decrease in the number of positive samples. However, in order to confirm this assertion other studies using different approaches are necessary, especially with regard to inactivation of alcohol gel for evaluation of efficacy.
Concurrent cleaning is a surface cleaning processes used in health care. In this process cleaning of all horizontal surfaces, furniture, and equipment that make up the inpatient unit of the patient should be carried out, especially those who have greater contact with the hands of the patient and staff, such as door handles, telephones, switches light, bed rails, bells to trigger the nursing team, among others12. In this context and based on the results observed in our study, it is believed that the recommendation for cleaning of alcohol gel bottles arranged in patient unit should receive special attention as the cleaning process as well as the other above-mentioned objects.
An National Study of Review of extrinsic contamination by dynamic liquid antiseptic soap and the use of the process in Brazilian hospitals identified a significant number (9.4%) of contaminated samples, antiseptics (70% alcohol, alcohol gel, chlorhexidine and povidone-iodine topical) accounted for 23.8% of contaminated samples. Among the antiseptics, alcohol products were the most frequently contaminated, the contamination mostly feature polymicrobial7. This result is similar to that obtained in this study in which we observed in a minority of samples isolated growth of a type of bacteria.
The same national study identified only two species of Gram+ ( Staphylococcus aureus and Staphylococcus epidermitis) and other Gram-7. In this study, as the Gram-, his presence was overestimated, and the results were not consistent with what was expected. The three isolates were gram-negative bacilli, classified as non-fermentative, aerobic microorganisms, not spored, which are characterized as incapable of using carbohydrates as a source of energy through fermentation, degrading them by the oxidation pathway. The characterization of this group of bacteria is of great importance in cases of infection related to health care, they usually show resistance to many antibiotics and are capable of causing serious infections. The isolated bacteria (Pseudomonas aeruginosa and Acinetobacter baumannii) colonize and cause infections, especially in severe patients undergoing invasive procedures and13,14.
A retrospective study that characterized the IRAS in a neonatal intensive care unit (NICU), noted that the main etiological agents isolated in blood cultures were Pseudomonas aeruginosa it's the Staphylococcus coagulant-negative. The majority presence of the latter in several studies may be related to contamination and sample handling, since these bacteria are naturally present in the skin of the patient and health care professionals15.
At the same time, other research that analyzed the main causes of preventable death by proper attention to the newborn showed that sepsis is the second leading cause of mortality in this population16.
Another unexpected result of research concerning the presence of bacteria in the alcohol gel and bottle at the time of installation of the same in the bed of the child, evidenced by culture obtained immediately after removal of the vial from inside the manufacturer's packaging. All alcohol bottles included in the survey came from the same manufacturing lot. According to interrogation conducted by ANVISA, the limits of acceptability of microbiological control for products such as alcohol gel are as follows: count of aerobic mesophilic microorganisms, no more than 103 Colony Forming Units (CFU) / g or ml (maximum limit: 5 x 103 CFU / g or ml); absence of Pseudomonas aeruginosa in 1g or 1ml; absence of Staphylococcus aureus in 1g or 1ml; and the absence of total coliforms and fecal in 1g or 1mL.
CONCLUSION
The use of contaminated antiseptics can constitute a frequent source of microorganisms responsible for outbreaks of infections in hospitals. Most of the cultures analyzed showed no bacterial growth. It was observed that the cover is where we most identify bacterial growth at all sampling times, and with advancing time, also the cover can be observed increase in the number of positive cultures was not observed other sites in the obtaining samples. There was a predominance of Gram+ bacteria.
Contaminated surfaces, as well as the hands of health professionals can contribute to cross-contamination of patients. Hand hygiene of health workers and cleaning and disinfecting of surfaces, equipment and devices located at the patient care area are key to the prevention and reduction of infections related to health care. Among the limitations of the study we highlight the use only of qualitative culture of samples. It is suggested that future research should therefore also include quantitative analysis.
REFERENCES
1. Ministério da Saúde (Br). Agência Nacional de Vigilância Sanitária. Segurança do Paciente em Serviços de Saúde: Higienização das Mãos. [Internet]. Brasília (DF): ANVISA; 2009. [cited 2016 April 1]. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/seguranca_paciente_servicos_saude_higienizacao_maos.pdf
2. Prado MF, Maran E. Desafio ao uso das preparações alcoólicas para higienização das mãos nos serviços de saúde. Esc Anna Nery. 2014;18(3):544-547.
3. World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. First global patient safety challenge. Clean care is safer care. [Internet]. Geneva (SUI): WHO; 2009. [cited 2016 April 2]. Available from: http://whqlibdoc.who.int/publications/2009/9789241597906_eng.pdf?ua=1
4. Padoveze MC, Fortaleza CMCB. Infecções relacionadas à assistência à saúde: desafios para a saúde pública no Brasil. Rev Saúde Pública [Scielo-Scientific Electronic Library Online]. 2014 [cited 2016 June]. 48: 995-1001. Available from: http://www.scielo.br/pdf/rsp/v48n6/pt_0034-8910-rsp-48-6-0995.pdf
5. Silva AS. Estudo das formulações e metodologias analíticas de saneantes domissanitários com ação antimicrobiana, de uso hospitalar, com registro em 2004 e 2005. [master degree]. Rio de Janeiro (RJ): INCQS/FIOCRUZ; 2008. [cited 2016 March 29]. Available from: http://teses.icict.fiocruz.br/pdf/3550_AdrianaSantanaDaSilva.pdf
6. Ministério da Saúde (Br). Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada – RDC nº 42, de 25 de outubro de 2010. Dispõe sobre a obrigatoriedade de disponibilização de preparação alcoólica para fricção antisséptica das mãos, pelos serviços de saúde do país e dá outras providências. [Internet]. Diário Oficial da União. Brasília (DF): ANVISA; 2010 [cited 2016 April 2]. Available from: http://www20.anvisa.gov.br/segurancadopaciente/index.php/legislacao/item/rdc-42-de-25-de-outubro-de-2010
7. Serufo JC. Avaliação da dinâmica de contaminação extrínseca de sabonetes líquidos e anti-sépticos no processo de uso em hospitais brasileiros da rede sentinela. [Internet]. Belo Horizonte (MG): Agência Nacional de Vigilância Sanitária (ANVISA); 2007. [cited 2016 April 29]. Available from: http://www.anvisa.gov.br/servicosaude/controle/anti_septicos_final.pdf
8. 8. Nóbrega HN, Ferreira JAB, Romão CMCPA, Capasso IRVF. Atividade antimicrobiana in vitro de produtos antissépticos por meio de técnica time kill. Rev Inst Adolfo Lutz. São Paulo, 2013; 72(3):226-33.
9. Santos AAM, Verotti MP, Sanmartin JA, Mesiano ERAB. Importância do álcool no controle de infecções em serviços de saúde. Rev Adm Saúde. 2002; 4(16):7-14.
10. Tavares LLP, Nascimento AE, Okada K, da Silva CAA. Seleção de diferentes meios para produção de lipase a partir de Bacillis licheniformis (UCP 1014). Exacta. 2011;9:309-16.
11. Pereira PMA, Castro EAR, Pereira JAA, Tórtora JCO. Resistência aos antimicrobianos em Estafilococos Coagulase-negativa isolados de hemocultura. J bras Med. 2007;93:26-9.
12. Ministério da Saúde (Br). Agência Nacional de Vigilância Sanitária. Segurança do Paciente em Serviços de Saúde. Limpeza e desinfecção de superfícies. [internet]. Brasília (DF): ANVISA; 2010. [cited 2016 April 3]. Available from: http://www.anvisa.gov.br/servicosaude/controle/anti_septicos_final.pdf
13. American Thoracic Society Documents. Guidelines for the management of adults with hospital acquired, ventilator–associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171:388-16.
14. Bhaumik P, Purav P, Payal R, Mitesh P, Piyush P, Mahendra V. Bacteriological profile and antibiogram of gram negative organisms isolated from medical and neurology intensive care unit with special reference to multi-drug resistant organisms. Natl J Med Res. 2012;2:335-8.
15. Oliveira COP et al. Caracterização das infecções relacionadas à assistência à saúde em uma unidade de terapia intensiva neonatal. Rev enferm UERJ. 2013; 21(1):90-4.
16. Gaiva MAM, Fujimori E, Sato APS. Mortalidade neonatal: análise das causas evitáveis. Rev enferm UERJ. 2015; 23(2):247-53.