Source: Reporting and investigation records.
(*) Other units that had a report: pharmacy, high cost pharmacy, surgical center, oncology pharmacy, endoscopy, clinical surgical center, and clinic.
RESEARCH ARTICLES
Drug-related incidents in a hospital: input to improving management
Flávia Fernanda Rosa D' AquinoI; Carmen Maria Casquel Monti JulianiII; Silvana Andrea Molina LimaIII; Wilza Carla SpiriIV; Carmen Silva GabrielV
I
Master's Degree in Nursing. Nurse from Lauro de Souza Lima Institute. Bauru, São Paulo, Brazil. E-mail: flaviarosa_enf@yahoo.com.br
II
PhD Degree in Nursing. Lecturer in the Nursing Department, School of Medicine of Botucatu. Sao Paulo, Brazil. E-mail: cjuliani@fmb.unesp.br
III
PhD Degree in Nursing. Lecturer in the Nursing Department, School of Medicine of Botucatu. Sao Paulo, Brazil. E-mail: smolina@fmb.unesp.br
IV
PhD Degree in Nursing. Lecturer in the Nursing Department, School of Medicine of Botucatu. Sao Paulo, Brazil. E-mail: wilza@fmb.unesp.br
V
PhD Degree in Nursing. Lecturer of the School of Nursing of Ribeirão Preto, University of São Paulo. Brazil. E-mail: cgabriel@eerp.usp.br
Article deriving from the Professional Master's Degree Dissertation Incidentes relacionados a medicamentos em uma instituição hospitalar: subsídios para a gestão. Author: Flávia Fernanda Rosa D' Aquino. School of
Medicine of Botucatu, 2014.
DOI: http://dx.doi.org/10.12957/reuerj.2015.10637
ABSTRACT
This retrospective, quantitative, descriptive study to identify drug-related incidents and their determinants described in spontaneous reports at a hospital in São Paulo State. The incidents were identified from 189 spontaneous reports filed with the Patient Safety Center from June 2011 to June 2012. The average rate of notifications per 1000 patient-days per month was 1.94. Medication errors were the incidents most reported. Error category, medication type, main failures and likely causes were recorded. Of total incidents, 19.8% caused no harm to patients, 8.6% caused harm (adverse events), and 60.4% were intercepted by nurses before affecting patients (potential adverse event). The results of this study provide input for professionals involved in the medication system to implement incident prevention strategies.
Keywords: Medication errors; hospital medication systems; safety management; patient safety.
INTRODUCTION
The occurrence of incidents that affect patient safety is a Global concern. World Health Organization (WHO) launched in 2004 the Global Alliance Program for the Safety of the Paciente1 that call on the countries to adopt strategies for improving the quality and care of patients.
In Brazil, the National Patient Safety Program was established by Ordinance No. 529/2013 of the Ministry of Health, with the aim of contributing to qualification in healthcare2 and, thus, preventing the incidents. That same year, the National Health Surveillance Agency (ANVISA) published RDC 36/2013, which established the actions to promote patient safety and the improvement of quality in healthcare services3.
Among the incidents that affect patient safety, Drug-related Incidents (DRI) deserve special attention from the hospital institutions, because epidemiological studies in the United States estimate that every patient in the hospital is subject to medication error per day and, approximately, 400,000 drug-related adverse events happen each year4.
To promote safe practices in using drugs and preventing DRI, the Ministry of Health created a security protocol on prescription, use, and administration of drugs containing strategies for monitoring the incidents5.
The monitoring of incidents can be accomplished by spontaneous report of the involved parties5, which is essential for improving patient safety, because this knowledge leads to evaluation of work processes in order to make them safer.
This article aimed to identify DRI and their determining factors in the reports sent to the Patient Safety Center (PSC) of a public hospital in the State of São Paulo.
LITERATURE REVIEW
The incidents are defined as circumstances that could have result or have resulted in unnecessary harm to the pacient6, and can be classified as incidents without harm (incident reached the patient, but did not cause harm), harming incidents (adverse event) or near miss (incidents intercepted before reaching the patient) considered a potential adverse event6,7.
Drug-related Incidents (DRI) are any irregularities in the process of using a drug and may occur at any stage of the drug system8. The nurses are legally responsible for preparing and administering drugs, the final step of this complex system that, if susceptible to faults, may determine the occurrence of DRI9,10.
The nurses, through their role, may identify shortcomings in drug preparation and administration and, with the help of other professionals, knowing the flaws in the whole drug system, so that, they may together pursue the prevention of incidents.
METHODOLOGY
This is a descriptive, retrospective study with quantitative approach that sought the incidents occurring in drug-related therapy of a public hospital in the State of São Paulo in Brazil.
The location of the study is considered a teaching hospital, with 318 beds and an average of 7346 patient-day a month, Level I Accreditation by the National Accreditation Organization (NAO), is part of Rede Sentinela (Sentinel Network) and is a regional referral service. It has eight inpatient units, an emergency unit, four intensive care units (adult, pediatric, burnt, and coronary), diagnostic and therapy unit, three surgical centers, material and sterilization center, and a renal substitutive therapy center.
We used all the spontaneous report records forwarded to the PSC in the period from June 2011 to June 2012, totalizing 189 records. The PSC is a sector, at the institution, which has as its objective the analysis of reported incidents and the causes and mitigation of incidents, through the adoption of strategies/barriers, for their prevention.
The data were collected by the investigator herself, in the first half of 2013, through access to the computerized system for spontaneous reports and the investigation records on incidents used by the PSC of that institution.
Data were tabulated using an ExcelÒ spreadsheet and submitted to statistical descriptive analysis in frequencies and percentages, using the program SAS for Windows 9.2.
The research was approved by the Research Ethics Committee (REC), according to official written notice 493/2012-CEP-Botucatu. Ethical procedures were followed, according to Resolution No. 466/2012.
The pharmacological groups were registered according to the Anatomical Therapeutic Chemical Classification (ATC) of drugs according to the anatomical therapeutic chemical system of the World Health Organization (WHO) - Collaborating Centre for Drug Statistics-Methodology11 and according to the list of high-risk drugs of the Institute for Safe Medication Practices (ISMP's)12, which by the National Agency of Sanitary Surveillance (ANVISA) are called potentially dangerous drugs (PDD).
RESULTS AND DISCUSSION
In total, 189 reports were sent to the PSC, which represented 1.94 average rate per 1000 patients-day a month
Another study analyzed medication errors in a pediatric hospital and showed 1.15 rate per 1000 patients-day13. Although the rate of this study is expressive compared to the literature, the comparison is limited due to the differing characteristics among hospitals. However, we may consider that this study had a low frequency of reports for being a high-complexity hospital, by the number of beds, for having four intensive care units, being an accredited hospital, and participating in Rede de Hospitais Sentinela (Sentinela Hospital Network), which could stimulate the reports.
Among the DRI, the medication errors wer the most identified - 116 (61.5%), followed by adverse reactions to drugs - 40 (21.2%), technical complaints - 22 (11.7%) and therapeutic non-effectiveness - 11 (5.6%). Other studies also pointed out the problems in drug-related therapy as being the most reported: 63% 14 and 71%15.
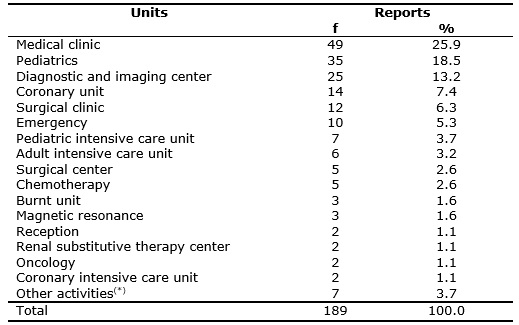
In relation to the origin of the reports, most reporting units were the medical clinic units 49 (25.9%) and 35 (18.5%) pediatrics. The frequencies of the reports per unit are arranged in Table 1.
TABLE 1:
Frequencies of reports for drug-related incidents per preceding unit. Bauru, SP, June 2011 to June 2012.

Source: Reporting and investigation records.
(*)
Other units that had a report: pharmacy, high cost pharmacy, surgical center, oncology pharmacy, endoscopy, clinical surgical center, and clinic.
In a study of spontaneous reports, the most reporting unit was also the medical clinic14. The occurrences in the pediatric units are expected, because the pediatric patients are three times more predisposed to medication errors and adverse events related to drugs in relation to the adult population for various factors, such as the different dosage forms of the same drug, solution concentration diversities, dosage depending on the weight of the patient and also on calculations of the own maturity of the organs that can hamper metabolism and excretion of drugs16.
The unit that more reports is not necessarily the one that has more incidents, because the one with smaller number may be underreporting. Thus, low frequencies of reporting does not mean secure systems, and can even mean the opposite, that is, as the teams are more prepared and aware to the problematic, they tend to report more.
Underreporting is an important aspect considered in health services and, for its reduction, reporting the occurrences must always be guided and encouraged by all the team17. Underreporting may occur due to various factors, such as fear, guilt, and kind of reporting system, being one of the main difficulties for the voluntary reporting method15.
The reported medication error categories were wrong dispensation - 56 (48.3%), wrong patient-16 (13.8%), wrong drug - 13 (11.2%), wrong dose- 10 (8.6%), wrong prescription - 9 (7.8%), wrong time -7 (6.0%), wrong application method - 3 (2.6%) and dose omission - 2 (1.7%). In comparison with other studies, the most present medication error category was dose omission18,19, which was not representative in this study.
The drug class more involved in medication error reports was systemic antibiotics, representing 22 (19%) records. The errors in antibiotic administration may result in microbial resistance. This research corroborates a Brazilian multicentric study that found 18.5%20 error rate in the administration of antimicrobials.
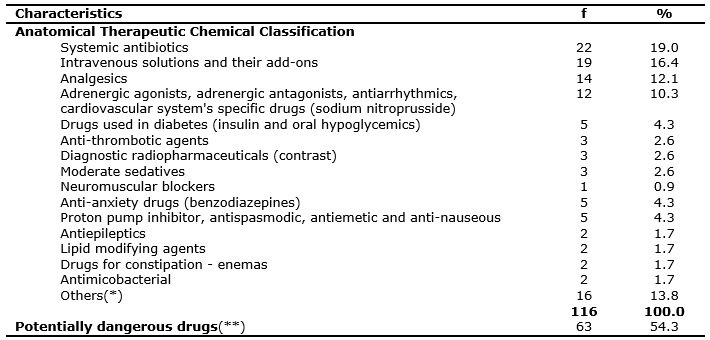
In this study, 63 (54.3%) reports covered drug classes that correspond to potentially dangerous drugs (PDD). As a matter of ranking, three of the reports involving liposomal form antibiotics were added to PDD. The characteristics of these drugs are given in Table 2.
TABLE 2:
Characteristics of drugs registered in the reports. Bauru, SP, June 2011 to June 2012.
Source: Reporting and investigation records.
(*) Others: anti-anemic, anti-hemorrhagic, anti-inflammatory, urinary antispasmodic, thyroid hormone, bronchodilator, antipodagric, dermatological
antiseptic, Alzheimer's disease drug, drugs without identification.
(**) Including three antibiotics in liposomal form.
A Brazilian study, which portrayed the PDD, revealed 37.4%19 rates. All medication errors may lead to harms to the patients, but the PDD described by the ISMP's have high risk for harms and the consequences can be devastating12, this makes these findings worrying.
Data from a study, carried out in Brazil, with 14 risk managers show that the less widely used initiatives for patient's safety are related to PDD control and prevention of drug-related adverse events21. The ISMP's directs the institutions to standardize on the steps of arrangement, storage, preparation and administration, improving access to information on the PDD, using auxiliary labels for identification, automatic alerts and double-check whenever necessary12.
Higher occurrence failures were dispensation of exchanged drugs - 28 (24.1%), dispensation of drug to wrong patient - 13 (11.2%) and administration of a drug in wrong patient - 12 (10.3%), followed by drug dispensation with volume that indicates wrong application method – 10 (8.7%), drug dispensation with wrong dose - 9 (7.8%), non-dispensed drug - 7 (6.0%), dispensation of drug with the label name different from the drug's name - 4 (3.4%), dispensation of drug with non-sterile cover - 1 (0.9%), dispensation of drug with strange body between the plunger and the syringe - 1 (0.9%), dispensation of expired drug - 1 (0.9%), exchange of name on the patient bands - 4 (3.4%), drug administered twice - 2 (1.7%), non-administered drug - 2 (1.7%), wrong dosage administration - 5 (4.3%), administered on the wrong time - 4 (3.4%), drug's wrong application method - 2 (1.7%), drug prescription for an allergic patient - 4 (3.4%), prescription of drug for the mother in the prescription of the patient/ child - 1 (0.9%), prescription at wrong place (pharmacy cannot see)-1 (0.9%), wrong-dose prescription-1 (0.9%), non-standard drug prescription - 1 (0.9%), printing an old prescription by the nursing staff - 2 (1.7%), drug not dispensed upon discharge from hospital by the nursing staff - 1 (0,9%).
Dispensation of exchanged drugs was related to drugs with similar names and sounds, similar ampoules and infusion solutions and their add-ons that were sent in concentrations different from the prescribed. Global authorities, pharmaceutical companies, and the institutions must confer more importance to these drugs, as an improvement in the patient safety22.
Some strategies are described by the World Health Organization (WHO) for preventing these errors, such as conducting an annual survey for drugs, guiding the professionals, minimizing requests per verbal orders, giving importance to reading the labels and not only their visual recognition and the drug's physical location, knowing the effect of the drug, ensuring the readability of the prescription including the generic name and pharmaceutical form of the drug, clarifying verbal orders before the administration, separating physically the drugs with similar names, using writing with uppercase and lowercase in prescribing these drugs and guiding the patients about their drugs so that they may recognize problems related to similar sounds and names in relation to their drug22.
In relation to drug dispensing to wrong patients, WHO guidelines for minimizing these errors are emphasizing the responsibility of the professionals in identifying the patients before any procedure, encouraging the use of at least two identifiers (for example: the name and date of birth), standardizing the approaches for identification (use of bracelets, identification to be held by the name and date of birth or using technology), standardizing approaches for patients with similar names, encouraging patients to take part in the entire process, encouraging the labeling of the used containers, continuous training for the professionals and encouraging family members to report errors23.
The most reported probable causes on the investigation records were double-checking failure of drugs in the pharmacy - 31 (26.7%), failure to adhere to drug-related guidelines -24 (20.7%) and communication failure - 10 (8.6%), followed by handling failure - 8 (6.9%), similar sounds and spellings and similar flask - 5 (4.3%), pharmacy followed the opinion of the infection control service instead of prescription - 5 (4.3%), pharmacy's labor problem - 4 (3.4%), pharmacy's routine changes -2 (1.7%), problems in the process of buying - 2 (1.7%), handwriting prescription - 1 (0.9%), drug tape opened simultaneously and drugs exchanged by the nursing staff - 1 (0.9%), a drug label stuck to another one - 1 (0.9%), a pill cannot be shredded, sent in a solution – 1 (0.9%), exchange of infusion pumps - 1 (0.9%), failure in the investigation of patient's allergy - 1 (0.9%), lack of awareness on the hazards by the physician (prescription for the mother in the patient's prescription) - 1 (0.9%), ignorance of the pediatric dosage - 1 (0.9%) and probable cause absent in the records - 17 (14.5%).
In the context of this study double-checking is an activity performed by pharmacy technicians, who are humans and liable to erros24. Automated systems can assist in controlling the wrong dispensation and decreasing medication errors. 25.
Another probable cause for medication errors was the failure on adhering to the institutional guidelines. A North American study also points out the problem of non-adherence to drug-related guidelines, indicating the need for more practical guidelines for drug administration; this factor is relevant for the clinical practice26. Knowing the process difficulties of the professionals for the safe performance and planning actions that facilitate the adherence to the guidelines must be permanent institutional actions.
Miscommunication was the third possible cause for medication errors described in this study's investigations. The communication process in the hospital is complex due to the number of professionals and the number of data and activities; the failures represent the lack of integrated processes and involve information transfer and acceptance for care responsibility27.
In relation to the incidents, 70 (60.4%) were intercepted by the nursing staff before reaching the patients (potential adverse event – near miss), 23 (19.8%) reached the patients, but did not cause any harms, complications or altered vital signals (incidents without harm), and 10 (8.6%) caused harms (adverse events) and in 13 (11.2%) reports, the records on the harms were absent. Six-year study in England showed that most incidents did not result in harms - 83%, 13% showed light harms, 0.15% caused severe harms and death18.
In this study, the nursing staff had an important role in intercepting 60.4% of the DRI, however, there is a need to strengthen the previous steps, because safe systems should intercept errors even before the drug administration. Drug administering step is considered vulnerable and errors are less likely to be intercepted in this fase28, because of the number of activities carried out, requiring several institutional protocols and guidelines 29.
CONCLUSION
This study allowed to identifying the DRI and their determining factors. Joint efforts of drug-system professionals should be directed towards the objective to reduce the incidents and improve patient safety culture.
Although the results demonstrate the importance of the nursing staff, which intercepted 60.4% of the errors, such data should be viewed with discomfort, because the nursing staff, which is overloaded in the hospital institutions, should not receive drugs at odds with the norms, because this condition increases the risk to the patients. Ideally, the previous checking should allow for the correct drug dispensation and identification.
REFERENCES
1.World Health Organization. World Alliance for Patient Safety: forward programme [site de Internet]. 2006-2007. Geneva (Swi). [cited in 2014 Jun 10] Disponível em: http://www.who.int/patientsafety/information_centre/WHO_EIP_HDS_PSP_2006.1.pdf .
2.Ministério da Saúde (Br). Programa Nacional de Segurança do Paciente. Portaria nº 529 de 1° de Abril de 2013. Institui o Programa Nacional de Segurança do Paciente. Brasília (DF): Gabinete Ministerial; 2013.
3.Ministério da Saúde (Br). Resolução - RDC nº 36, 25 de julho de 2013. Institui ações para a segurança do paciente em serviços de saúde e dá outras providências. Brasília (DF): Gabinete Ministerial; 2013.
4.Institute of Medicine. Preventing medication errors: quality chasm series. Washington (DC): National Academy Press; 2006.
5.Ministério da Saúde (Br). Protocolo de segurança na prescrição, uso e administração de medicamentos. Protocolo integrante do Programa Nacional de Segurança do Paciente. Brasília (DF): Ministério da Saúde; 2013.
6.World Health Organization. The conceptual framework for the international classification for patient safety. Version 1.1. [site Internet]. WHO; 2009. [cited in 2014 jun 10] Available from: http://www.who.int/patientsafety/taxonomy/icps_chapter3.pdf.
7.Capucho HC. Near miss: quase erro ou potencial evento adverso? [Carta ao editor]. Rev Latino-Am Enfermagem. 2011;19:[2 telas]. [citado em jul 2014] Disponível em: http://www.scielo.br/pdf/rlae/v19n5/pt_27.pdf.
8.Morimoto T, Gandhi TK, Seger AC, Hsieh TC, Bates DW. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13:306-14.
9.Cassiani SHB, Miasso AL, Silva AEBC, Fakin FT, Oliveira RC. Aspectos gerais e número de etapas do sistema de medicação de quatro hospitais brasileiros. Rev Latino-Am Enfermagem. 2004;12:781-9.
10.Oliveira RB, Melo ECP. O sistema de medicação de um hospital especializado no Município do Rio de Janeiro. Esc Anna Nery. 2011; 15:480-9.
11.World Health Organization. Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment 2013 [site Internet]. Oslo, 2012. [cited in 2014 Mai 03] Available from: http://www.whocc.no/filearchive/publications/1_2013guidelines.pdf.
12.Institute for Safe Medication Practice. ISMP's list of higth-alert medications [site Internet]. [cited in 2014 Nov 30] Available from: http://www.ismp.org/tools/highalertmedications.pdf.
13.Yamamoto MS, Peterlini MAS, Bohomol E. Notificação espontânea de erros de medicação em hospital universitário pediátrico. Acta Paul Enferm. 2011; 24:766-71.
14.Bezerra ALQ, Silva AEBC, Branquinho NCSS, Paranaguá TTB. Análise de queixas técnicas e eventos adversos em um hospital sentinela. Rev enferm UERJ. 2009; 17:467-72.
15.Capucho HC, Arnas ER, Cassiani SHB. Segurança do paciente: comparação entre notificações voluntárias manuscritas e informatizadas sobre incidentes em Saúde. Rev Gaúcha de Enferm. 2013;34:164-72.
16.Gonzales Kelly. Medications administration errors and the pediatric population: a systematic search of the literature. Journal of Pediatric Nursing. 2010;25:555-65.
17.Lima PF, Cavassini ACM, Silva FAT, Kron MR, Gonçalves SF, Spadotto A, et al. Queixas técnicas e eventos adversos a medicamentos notificados em um hospital sentinela do interior de São Paulo, 2009-2010. Epidemiol Serv Saúde. 2013; 22:679-86.
18.Cousins DH, Gerrett D, Warner B. A review of medication incidents reported to the National Reporting and Learning System in England and Wales over 6 years (2005-2010). Br J Clin Pharm. 2011;47:597-604.
19.Silva AEBC, Reis AMM, Miasso AI, Santos JO, Cassiani SHB. Eventos adversos a medicamentos em um hospital sentinela do Estado de Goiás, Brasil. Rev Latino-Am Enfermagem. 2011; 19:[9 telas].
20.Marques TC, Reis AMM, Silva AEBC, Gimenes FRE, Opitz SP, Teixeira TCA et al. Erros de administração de antimicrobianos identificados em estudo multicêntrico brasileiro. Rev Bras Ciências Farm. 2008; 44:305-14.
21.Souza RFF, Silva LD. Estudo exploratório das iniciativas acerca da segurança do paciente em hospitais do Rio de Janeiro. Rev enferm UERJ. 2014; 22:22-8.
22.World Health Organization. Patient safety solutions. 2007 [site Internet]. [cited in 2014 Dec 07] Available from: http://www.who.int/patientsafety/solutions/patientsafety/PS-Solution1.pdf.
23.World Health Organization. Patient safety solutions. 2007 [site Internet]. [cited in 07 dez 2013] Available from: http://www.who.int/patientsafety/solutions/patientsafety/PS-Solution2.pdf .
24.Reason J. Human error: models and management. BMJ 2000; 320:768-70.
25.Chapuis C, Roustit M, Bal G, Schwebel C, Pansu P, Tchouda SD et al. Automated drug dispensing system reduces medication errors in an intensive care setting. Crit Care Med. 2010; 38:2275-81.
26.Kim J, Bates DW. Medication administration errors by nurses: adherence to guidelines. J Clin Nurse. 2012; 590-8.
27.Agência Nacional de Vigilância Sanitária (ANVISA). Assistência segura: uma reflexão teórica aplicada a prática [site de Internet]. Ministério da Saúde; 2013. [citado em 15 out 2013] Disponível em: http://www20.anvisa.gov.br/segurancadopaciente/images/documentos/livros/Livro1-Assistencia_Segura.pdf .
28.Leape LL, Bates DW, Cullen DJ, Cooper J, Demonaco HJ, Gallivan T et al. Systems analysis of adverse drug events. JAMA.1995; 274:35-43.
29.Jennings BM, Sandelowski M, Mark B. The nurse's medication day. Qualitative Health Research. 2011; 21:1441-51.