RESEARCH ARTICLES
Management adverse events following immunization for nursing team: care challenges
Hayda AlvesI; Ligia Maria Gomes DomingosII
INurse. PHD in Public Health, National School of Public Health Sergio Arouca, Oswaldo Cruz Foundation. Professor of Undergraduate Program in
Nursing, Humanities and Health Institute, Rio das Ostras Universtity Center. Fluminense Federal University. Rio das Ostras, Rio de Janeiro, Brazil. E-mail: halves@puro.uff.br
IINurse. Fluminense Federal University. Rio das Ostras, Rio de Janeiro, Brazil. E-mail: ligiamgd@gmail.com
ABSTRACT
The proper management of adverse events following immunization (EAPV) is an important aspect of safe use of vaccines. This study analyzed how be nursing
staff from a health care center develops their care practices related to management of mild, moderate and severe EAPV among children under one year old. It
is an exploratory, descriptive, qualitative approach study. Data was gathered in with 7 nursing from a reference vaccination service by participant
observation, document analysis and structured interviews in Macaé/RJ, in june 2010. The categorical analysis was used to treat. The results suggest:
inappropriate epidemiological surveillance and a restricted role of the nursing staff in the management of EAPV. Such challenges have hindered the
development of care by nursing staff immunization practice.
Keywords:
Vaccination; primary care nursing; epidemiological surveillance; public health.
INTRODUCTION
Vaccines, like any pharmaceutical product, are not free of side effects or adverse events that can affect vaccinated population1. Several
studies have discussed the importance of surveillance systems of adverse event post-vaccination (PVAE), since the safety of vaccines is crucial to expand
the membership of the general public to immunization programs. The use of large-scale immunobiologicals is a current and important strategy in the context
of practices in collective health1,2.
However, even if the immunobiological safety is guaranteed, the success of immunization programs, through the mass vaccination, creates a paradoxical
situation. As the perception of risk of immunobiological diseases declines, increases PVAE fears2,3. For this cause, diseases already controlled
and/or eliminated may return as a function of changes in population membership to the vaccination practice2,4-6.
Therefore the expansion and the continued success of immunization programs requires the improvement of surveillance practices relates to PVAE as well as
the proper management of these events. This strategy is important to ensure the safety and reliability of these programs4 and promote a positive
social reaction to the vaccination practice7 .
Thus, this study aimed to analyze how nursing staff of a specialist center handless light, moderate and severe PVAE with children up to one year old. In
this way, it is intend to discuss how the actions of surveillance and nursing care produced in vaccine rooms have contributed to the safe use of
immunobiologicals.
THEORETICAL FRAMEWORK
Despite the amplitude and effectiveness of actions of the Immunizations National Program (INP) on prevention and control of immune preventive diseases in
Brazil since the end of the decade of 1970s, the first initiative related to the monitoring and management of PVAE occurred only 20 years after the program
creation8.
In 1992, a Technical Advisory Committee on Immunizations organized the first protocols for investigation of possible adverse events. In 1998, with the
publication of the Handbook for the Epidemiological Surveillance of Adverse Events after Vaccination, this activity became more systematic throughout the
country. Only since the year 2000, was deployed the Surveillance Information System of Post-vaccination Adverse Events (SI-PVAE) and the information from
the states and municipalities could be collected and analyzed more comprehensively and consistently8.
In order to reinforce the importance of monitoring on adverse reactions from vaccines, in 2005 such events were classified as reportable diseases 1,8.
In 2009 the Programming of Actions and Health Surveillance (PAHS) established as one of its goals the notification and follow-up of 100% of severe PVAE. It
is worth noting, according to Ministerial Decree GM nº 3.008, December 1st, 2009, PAHS is a guiding axis to the achievement of goals of the Pact
for Life and other relevance priorities to the National Health Surveillance System and Health Surveillance at the federal, state and municipal areas 1. Even with the installation of the SI-PVAE several challenges remain related to the diagnosis, notification and proper running of PVAE.
Despite the Health Ministry to standardize guidelines and flowcharts, issues related to the technical capacity of the team that manages immunobiologicals,
infrastructure and organization of health services can cause problems and commit this type of assistance1,8.
The PVAE can be defined as: "any undesirable occurrence in individual who has received some immunobiological"8:24. Such events are primarily
classified as its extension, in local or systemic, and according to its intensity. The last aspect, they are characterized from three groups: light (when
does not require complementary test and/or medical treatment); moderate (when needing medical evaluation and complementary examinations and/or medical
treatment, not including in the severe category), severe (when triggers hospitalization for at least 24 hours; significant disability or dysfunction and/or
persistent (sequel); results in congenital anomaly; causes the need for immediate intervention to prevent death)8.
The events are also characterized in relation to vaccine (strains types, stabilizing substances and/or conservative, manipulation, conservation and
administration) and in respect of vaccinated (predisposing factors and/or immunologically idiosyncratic)1.
More than 30 types of PVAE are listed and codified to guide the conduct and feed the information system. The vast majority of events are light systematic
and local, however, because of the severity of them, surveillance actions are more oriented towards the moderate and seevere adverse events.8.
From the suspected case it must fill out the notification/research form and forward the instrument for local or municipal epidemiological surveillance. The
research should be carried out in 48 hours. From local level the notification follows to the regional, state and national levels1,8.
Adverse events occur according to the type of immunobiological used, the administration route, management, or the condition of the vaccines. In this
respect, the Health Ministry recommends functions to be performed by the health team at the local level as: to identify, investigate and notify the
occurrence of PVAE to Immunization Coordination and/or Surveillance Service of the municipality; to guide the vaccinated, family and/or guardians, to adopt
the relevant clinics conduct and to consolidate and analyze notified cases1,8.
In view of the importance of nursing care for the various health practices, including ensuring the proper functioning of the SI-PVAE becomes relevant to
analyze how this role has been played by the nursing staff in this context, so, how the caregiver dimension in production of health9 has been
developed from the vaccination practice.
METHODOLOGY
This is an exploratory descriptive study of qualitative approach conducted in the municipality of Macaé, located in the northwest of the state of Rio de
Janeiro.
For obtaining information were employed three procedures: participant observation, interviews and documentary analysis.
Participant observation10 was held at immunization center reference of the municipality in which the study was carried out. This phase lasted
from March to June 2010. During this period, the researcher remained in the service one day a week during its opening hours, recording information related
to the study object in a field notebook.
It was employed the technique of structured interview10,11 with service workers, applying a collection instrument with closed and open
questions. Instrument pre-test was conducted in an immunization service of a municipality coastal lowlands fluminense with similar characteristics to those
of the study place.
In June 2010, seven workers were interviewed from vaccines room that exercised its activities in that sector. In addition to this criterion, the subjects
selection was the willingness to participate in the study.
The average duration of the interviews was about an hour. They were held in the respondent's workplace and occurred with permission and signature of Free
Informed Consent. All the interviews were recorded and subsequently transcribed for analysis. The anonymity of the respondents was maintained and subjects
identification was performed from the use of E letter, followed by the subsequent numbers of interviews order realization.
For collected material treatment, was employed the categorical analysis technique. For this purpose, it aimed to understand the information via empirical
categories elaboration, built by declared elements by social group and/or perceived by the researcher, interpreted from a broader framework of theoretical
reality understanding11.
In documentary analysis10, it were surveyed PVAE notification of the municipality available on Immunizations and Coordination on Epidemiological
Surveillance in the municipality. Given difficulties in obtaining information about PVAE on a local level, this documentation was requested to the general
Coordination of Immunization Program (INP) the State Health Secretary and Civil Defense of Rio de Janeiro (SESDEC). The SI-PVAE notification Report of
Macaé municipality12, consolidated with information from suspected cases and closed of PVAE in the municipality, was kindly provided by the
central level and subsequently analyzed by researchers.
The project was approved by the Research Ethics Committee of the Medical School/HOSPITAL Antonio Pedro from Fluminense Federal University in 04/16/2010,
protocol nº 0.807.0.000.258-10.
RESULTS AND DISCUSSION
Interviewee Characterization
Seven subjects were interviewed, all female. Two of them were nurses the other technical training in nursing. Two interviewed worked in specialty center
for about four years, three exercised their functions in service of nine to ten years and the other for over 18 years.
PVAE notifications in Macaé/RJ
During the fieldwork, there have been several reports of nursing service workers investigated about the management of moderate and severe adverse events,
which demanded hospital outpatient or forwarding to infectious disease.
Despite an intense search of the information systems of epidemiological surveillance of Macaé were not found data on the PVAE notification.
On Coordination of Immunization Program of Macaé were found archived two forms for notification of suspicion of PVAE, which were unknown for
epidemiological surveillance of the municipality, but also were not compiled in SI-PVAE12.
The data in the SI-PVAE of the municipality were found only in the sector of Health Surveillance of the General Coordination of the INP of the State
Secretary of Health and Civil Defense of Rio de Janeiro (SESDEC), which is still a centralized system whose data are not available online at the
Informatics Department of the SUS (DATASUS) as vaccine coverage data13. The notifications were collected directly from the Report of the SI-EAPV
notification forms12 for the period from 1/1/2002 to 6/24/2010, as shown in Figure 1. It is worth noting that all the suspected cases were
confirmed and evolved to cure without sequels, according to notification information.
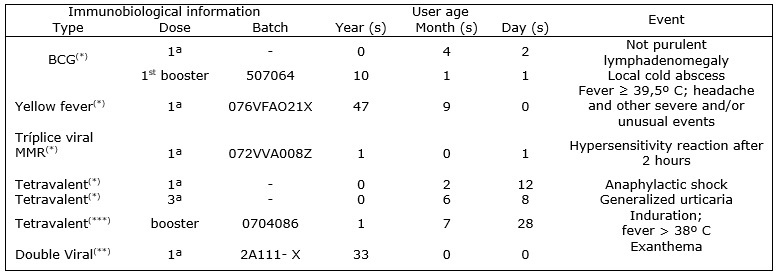
Legend: (-): not informed; BCG: Bacillus Calmette-Guérin or vaccine against tuberculosis; Tetravalent: diphtheria vaccine, tetanus, pertussis and haemophilus influenzae; Double viral: measles and rubella vaccine; MMR: vaccine against measles, mumps and rubella.
Sources: (*) The SI -PVAE Report13. (**)PVAE Certificate of Immunization Program Coordination of Macaé.
FIGURE 1: PVAE information. Macaé, Rio de Janeiro, Brazil, 2002 to 2010
It highlights that despite the system run since 2002, only from 2004 is information about the municipality of Macaé in SI-EAPV12.
According to information from consolidated doses of vaccinations in the municipality of Macae, available in the Information System and Evaluation of the
Program of Immunizations-SI-API, from 2002 to 2009 were vaccinated nearly 65 thousand children under the age of one year old13. Of these, 64825
were immunized against diphtheria, tetanus, whooping cough and haemophilus influenzae type B (Tetravalent vaccine), one of the most teratogeneric
immunobiologicals13-15.
From an observation at 10 000 vaccinated Basic Health Unit (BHU) in Teresina/Piauí, were verified 73 cases of PVAE classified as moderates and severe. Most
of the events occurred among children, in particular, the tetravalent vaccine (63.0%)14. Taking by reference this study, it is estimated that
approximately 298 EAPV Tetravalent related notifications between children under one year old should have been conducted in 2002 to 2010 in Macaé
municipality.
The data on PVAE municipality suggest therefore a sub notification framework, especially with regard to child vaccination. Also, it is noted the lack of
information on some forms, that committed, in a large-scale, the quality of the information produced15,16.
PVAE management by nursing stuff
The data about the management of PVAE by the nursing staff will be presented from the axes which guided the collection and analysis of information. In
Figure 2, these axles have been consolidated in the face of the main analytical categories about the management of PVAE, built from the talks of
respondents.

FIGURE 2: Axes for care and analytical categories on PVAE. Macaé, Rio de Janeiro, Brazil, 2010.
Guidance to the families of vaccinated children
The first category that emerged from the analysis of lines, vaccine reaction is simple, suggests that the nursing staff referred to teratogenic
potential of vaccines as manifestations of limited clinical repercussions and similar for different children, as highlighted in the statement:
What the child can have is local pain or fever, know know? You have to observe and make the antipyretic on the child. Ice in the area and observe.
(E4)
In this way, the second category appears. All the lines indicated a perception that all vaccine has the same reaction, which demand the same care –
another analytic category. This perception produced in the daily life of service was reproduced to users that the team offered guidance to the families of
the children vaccinated, as exemplified in the statement:
We manage the same thing over and over again, perform antipyretic if the patient has fever [...] ice compress to reduce the edema and myalgia on the
area.
(E5)
Through the analysis of the guidelines offered to families is suggested nursing care linked to management have been limited, in particular, on the moderate
and severe PVAE despite the possibility of their occurrence.
It was also found that the PVAE are treated largely as unusual events as highlighted by E7, and not as a real risk or as important sign referred to by the
caregiver(s) of the child vaccinated and who demand listen, objective and subjective analysis of the complaint and nursing interventions.
It is always the same. Suspected reaction happens, but in most cases it is not. But when it is a very strong reaction in the child, we show to the
nurse manager and she forwards to the doctor
(E7)
In another study, concerning the vaccination practice, in particular PVAE handling, care management held in a vaccine room, should take into consideration
that elements are not restricted to basic physiological effects of vaccines, but incorporating historical, cultural and religious aspects concerning the
immunobiologicals use17.
It is necessary to understand that the care produced by the health team in the management of PVAE and that the perception of risk of this type of event by
users is influenced both by the different effects of the teratogenic components of vaccines, and the health status of the subject, as individual
characteristics, and also by subjective aspects such as belief linked to use of certain vaccine and its possible reactions17.
Professional performance and the management of suspected PVAE
Only one of the respondents declared not to know the functioning of SI-PVAE. When asked to the professional how he acted in cases of suspected vaccine
reaction in a young child, the responses showed, by consensus, that the function of nursing regarding PVAE is observing the event and referral to another
professional or a new level of attention. In this sense, the analysis of lines brought out the third category What nursing does is observe or forward ... as highlighted by:
A gente encaminha para o infectologista para saber o que causou essa reação.
(E4)
It depends on the reaction. When is a local reaction, we guide to putting ice, which is a manufacturer's guidance. But when is a differentiated
reaction, like, a skin reaction, something like that, we forwarded to the pediatrician.
(E1)
To observe, to observe until it goes down [the reaction].
(E2)
We forwards to the infectious disease to find out what caused this reaction.
(E4)
Among those interviewed, the idea that nursing does very little, apart from (watch and forward), disregards the very guidelines exempt by nursing staff of
INP users, even though they are limited.
The speeches indicate that the clinical conduct referred by the team did not contemplate fully the guidelines contained in the Manual of PVAE8.
There is need for qualification of workers of nursing of the vaccine room with immunobiological management views according to the norms established by the
INP, as well as to conduct properly moderated and severe PVAE notification14,16. However, certain talks show another reality:
No, I've never done it. Not qualifying events.
(E1)
I started the vaccination activities in 1992 here at the free health center [...] and I never did a course of adverse event.
(E2)
This scenario indicates the need for interventions that enable the development of an integral care related to the vaccination practice. That is, permanent
education initiatives are needed on PVAE directed at professionals working in immunization rooms.
PVAE in vaccine room
About the PVAE that occur in the immunizations room immediately after the application of vaccines, it can be checked the fourth and fifth analytical
categories: It doesn't happen with me! and when we call someone ...
Many immediate reactions are not qualified or understood by the team as a PVAE that demands an specific attention. This reveals challenges for adequacy of
nursing practice technical standards for this care8. Here are the reports:
I don't do anything. Adverse reaction never occurred with me. If it happens in my hand ... [...] an anaphylactic reaction. It usually happens the
fainting, with teenager. Nothing ever happened immediately [...]. The only thing that happens is when the child is allergic to any component of the
vaccine, neomycin, antibiotics, vaccines preservative.
(E2)
It never happened to me. But if it had happened, I would call the nurse so she could see the child and make the necessary procedures.
(E7)
Both the nursing staff of the vaccines room as the technical manager for health surveillance actions at the local level has a preponderant role for the
functioning of PVAE surveillance system. From the proper management of these events, it becomes possible to contribute to the safe use of vaccines, in
order to enhance the achievement of care by qualified staff, as well as, to promote the construction of a health care that reflects the demands of society.
That is, to develop a health work clinically involved and centered in the sunject9.
In this perspective, the nursing staff requires careful practices build in vaccines rooms linked and extended family care exercised in the domestic sphere,
thereby enlarging the family protection measures and health promotion from child18.
CONCLUSSION
The results show a sub notification picture of PVAE and the weaknesses in the proper functioning of the SI-PVAE, which can compromise the safe use of
vaccines and the development of an integral care surrounding the vaccination practice.
It is necessary to enhance the role played by the nursing staff for the vaccination activity and, consequently, in the PVAE handling. These workers need
institutional investments relating to qualification and development assistance protocols in regard to determinations of INP.
Finally, the authors highlight the need of other studies that allow the analysis of infrastructure services and standardization of institutional protocols
for this assistance at the local level, but also on the knowledge of nursing stuff about PVAE management.
REFERENCES
1.Ministério da Saúde (Br). Secretaria de Vigilância em Saúde. Guia de vigilância epidemiológica. 6ª ed. Brasília (DF): Ministério da Saúde; 2005.
2.Centers for Disease Control and Prevention. Ten great public health achievements - United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999; 48:241-3
3.Chen RT, Stefano F. Vaccine adverse event: causal or coincidental? Lancet. 1998; 351:611-2.
4.Ellenberg SS, Chen RT. The complicated task of monitoring vaccine safety. Public Health Rep. 1997; 112:10-20.
5.Omer SB, Salmon DA, Orenstein WA, De Hart P, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J
Med. 2009; 360(19):1981-8.
6.Martins RM, Maia MLS. Eventos adversos pós-vacinais e resposta social. Hist. cienc. saude-Manguinhos. 2003; 10:807-25.
7.Smith A, Yarwood J, Salisbury DM. Tracking mothers' attitudes to MMR immunisation 1996-2006. Vaccine. 2007; 25:3996-4002.
8.Ministério da Saúde (Br). Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica. Manual de vigilância epidemiológica de eventos
adversos pós-vacinação. Brasília (DF): Ministério da Saúde; 2008.
9.Merhy EE. A perda da dimensão cuidadora na produção de saúde: uma discussão do modelo assistencial e da intervenção no seu modo de trabalhar a
assistência In: Merhy EE. Sistema Único de Saúde em Belo Horizonte- Reescrevendo o Público. Editoria Xamã, São Paulo; 1998. p.121-42.
10.Minayo MCS. O desafio do conhecimento: pesquisa qualitativa em saúde. 8ª ed. São Paulo: Hucitec; 2004.
11.Marconi MA, Lakatos, EM. Técnicas da pesquisa: planejamento e execução de pesquisas, amostragens e técnicas de pesquisas, elaboração, análise e
interpretação de dados. 6ª ed. São Paulo: Atlas; 2007.
12.Secretaria de Estado de Saúde e Defesa Civil do Estado do Rio de Janeiro. SESDEC. Relatório de Eventos Adversos Pós-Imunização. Macaé, (RJ): SESDEC;
2010.
13.DATASUS. Tecnologia da Informação a Serviço do SUS. [citado em 01 set 2010] Disponível em: http://tabnet.datasus.gov.br.
14.Araújo TME, Carvalho PMG, Vieira RDF. Análise dos eventos adversos pós-vacinais ocorridos em Teresina. Rev Bras Enferm. 2007; 60:444-8.
15.Freitas FRM. Vigilância de Eventos Adversos associados a vacina DPT e Preditores de Gravidade. Estado de São Paulo, 1984-2001[dissertação de mestrado].
São Paulo: Faculdade de Saúde Pública da Universidade de São Paulo, 2005.
16.Jesus DM, Bastos MA, Carvalho EC. Estudo dos eventos adversos provocados pela vacina tetravalente. Rev enferm UERJ. 2004; 12:299-305.
17.Spier RE. "Perception of Risk of Vaccine Adverse Events: A historical perspective." Vaccine. 2002; 20:S78-S84.
18.Figueiredo GLA, Pina JC, Tonete VLP, Lima RAG, Mello DF. Experiências de famílias na imunização de crianças brasileiras menores de dois anos. Rev
Latino-Am Enfermagem. 2011; 19:598-605.

